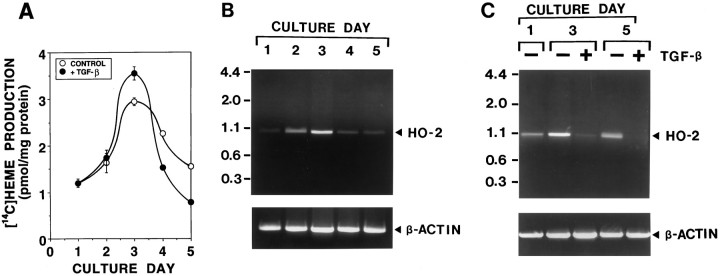
Fig. 4.
The change of heme biosynthesis and degradation during neuronal differentiation in primary cultures of olfactory receptor neurons. Primary cultures of olfactory receptor neurons were maintained in a feeding medium with and without 10 ng/ml TGF-β2 for 5 d. The effects of neuronal maturation, which are accelerated by TGF-β2 (olfactory neurogenic factor), on [14C]heme biosynthesis (A) and HO activity (B,C) were determined in cultures sequentially over time, as cells matured. A, The time course of [14C]heme biosynthesis during neuronal differentiation and the effect of TGF-β2. Cultures were fed with a medium in the presence (•) or absence (○) of 10 ng/ml TGF-β2. On each culture day, cultures were incubated with [14C]glycine to label heme. At 6 hr after addition of [14C]glycine, [14C]heme resulting from heme metabolism was measured in these cultures. B, The time course of HO-2 mRNA expression during neuronal differentiation. On each culture day, poly(A) RNA was prepared from the cultures. Oligo(dT)-primed cDNA was amplified by PCR, using HO-2-specific primers (top) and β-actin-specific primers (bottom) for 30 cycles. Under conditions used, PCR products increased linearly between 28 and 33 cycles. The predicted size of PCR products of HO-2 is 1033 bp. Similar results were obtained in three independent experiments (Band C are the results obtained from the independent series of culture preparations). C, The effect of TGF-β2 on HO-2 mRNA expression during neuronal differentiation. Cultures were fed with a medium in the presence (+) or absence (−) of 10 ng/ml TGF-β2. On each culture day, poly(A) RNA was prepared from these cultures, and PCR amplifications were performed for 30 cycles with HO-2-specific primers (top) and β-actin-specific primers (bottom). The same results were repeated in two independent experiments.