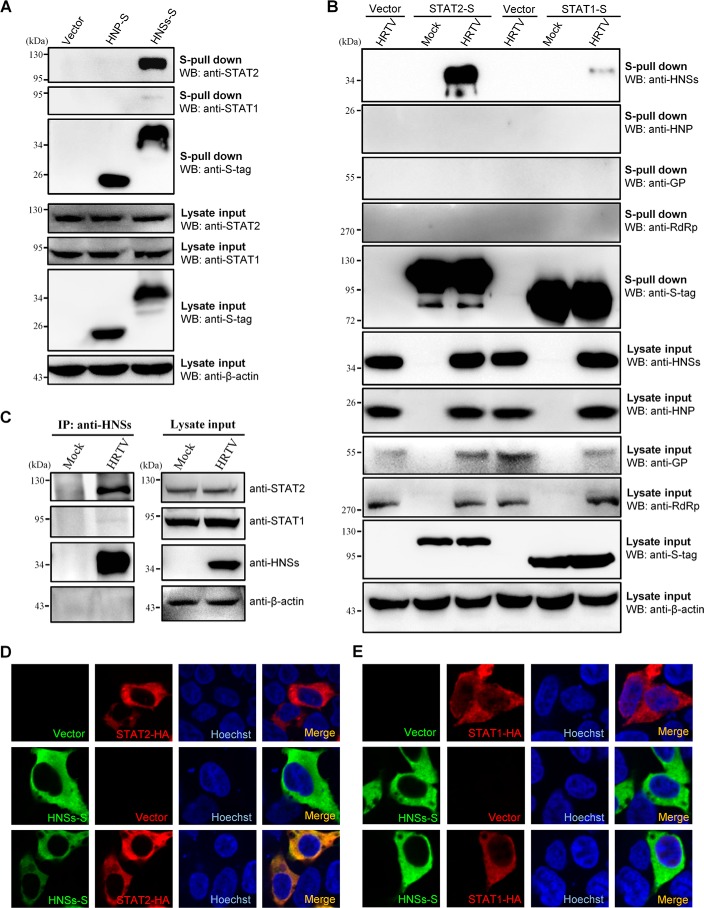
Figure 4.
Interaction and colocalization of HNSs with STAT2. A, HEK293 cells were transfected with the plasmids encoding S-tagged HNP (HNP-S) or HNSs (HNSs-S) or the vector plasmid. At 36 h posttransfection, cells were harvested for the S-pulldown assay. S-pulldown products and cell lysates were subjected to WB analyses with the indicated antibodies. B, HEK293 cells were transfected with the plasmids expressing S-tagged STAT2 (STAT2-S) or STAT1 (STAT1-S) or the control vector. At 12 h posttransfection, cells were mock-infected or infected with HRTV (MOI = 5) for 36 h and then lysed for the S-pulldown assay and WB analyses. Therein, GP expression was monitored with the detection of GN (the N-terminal region of GP) using anti-GN antibody. C, mock- or HRTV-infected HEK293 cells were lysed for a co-IP assay at 36 hpi. The lysate supernatants were first pretreated with preimmune serum and protein A/G–agarose and then used for co-IP with the HNSs-specific antiserum. Immunoprecipitates (IP) and cell lysates were delivered to WB analysis using the indicated antibodies. D and E, HEK293 cells were transfected with the plasmids expressing the indicated protein or the control vector. At 36 h posttransfection, cells were fixed for IFA to visualize the indicated proteins (HNSs in green and STAT2/STAT1 in red) by confocal microscopy. Nuclei were stained with Hoechst, as shown in blue.