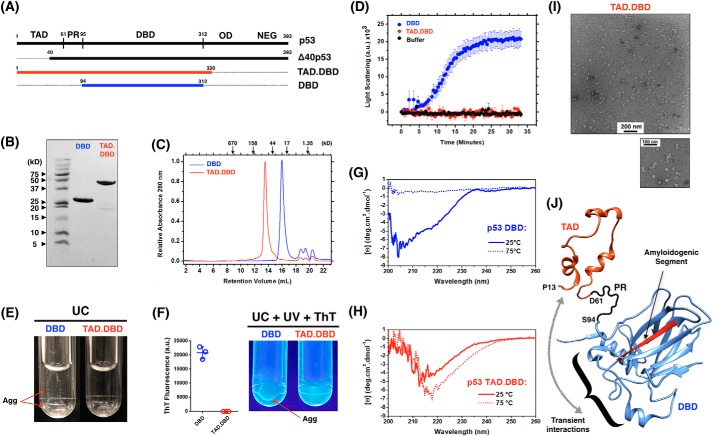
Figure 4.
The transactivation domain is a negative regulator of p53 aggregation. A, schematics of the TAD.DBD (red) and DBD (blue) constructs recombinantly expressed in this work. B, 20% SDS-PAGE stained with Coomassie Brilliant Blue showing purified proteins. C, analytic size-exclusion chromatography of both constructs monitored by absorbance at 280 nm. D, time course measuring the light scattering of p53 TAD.DBD and DBD at 37 °C. Results are shown as the average of three independent experiments. E, UC tubes showing DBD aggregation and a clear solution of the TAD.DBD construct. F, UV light illumination of ThT-bound DBD aggregates (Agg) after incubation at 37 °C for 40 min and UC. ThT fluorescence was quantified and is expressed as the average ± S.E. (n = 3). G and H, far-UV CD spectra of DBD (G) and TAD.DBD (H) at room temperature and at 75 °C. I, transmission EM of TAD.DBD species formed after heating the sample to 75 °C. J, atomic model representation of the TAD (PDB code 2L14, residues 13–61) and the DBD (PDB code 2FEJ, residues 94–297) to illustrate the negative regulation of the TAD segment over p53 DBD aggregation. The amyloidogenic segment of the DBD is colored red. PR, proline region; NEG, negative regulatory domain. The curly bracket shows the p53 DNA-binding motif in which the TAD potentially interacts according to Ref. 11.