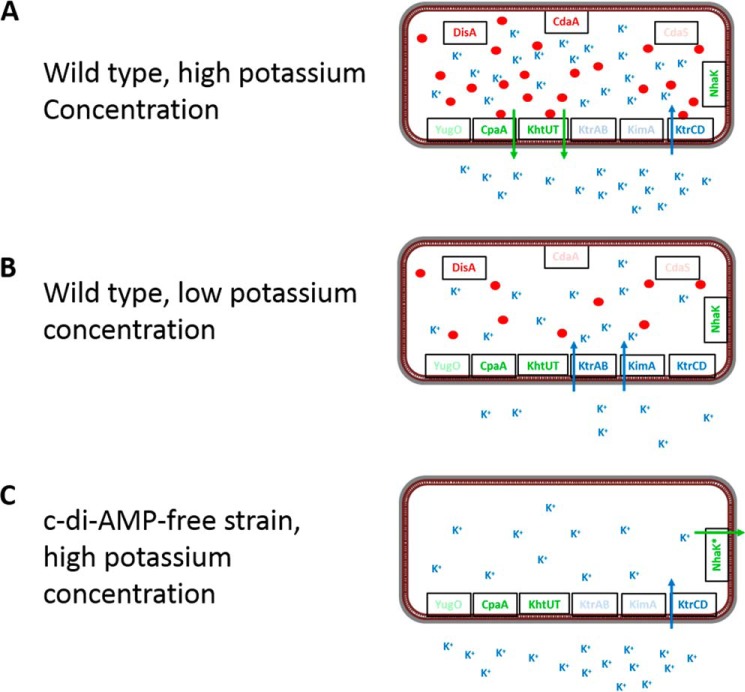
Figure 5.
Control of potassium uptake and export in B. subtilis by c-di-AMP. A, in the WT strain, the potassium exporters CpaA and KhtUT, as well as the low-affinity transporter KtrCD, are expressed at high potassium concentrations. Potassium is taken up by KtrCD and triggers the production of c-di-AMP by CdaA and DisA. Upon accumulation of potassium and the second messenger, c-di-AMP binds to KtrC to prevent further potassium uptake. Simultaneous binding of c-di-AMP to CpaA and KhtUT triggers potassium export. Thus, the cell prevents potassium intoxication. B, at low potassium concentrations, all three uptake systems as well as the two exporters are expressed. Potassium is transported by the high-affinity transporters KtrAB and KimA. The low intracellular potassium concentration results only in a very limited synthesis of c-di-AMP that is not sufficient to inhibit or activate potassium uptake or export, respectively. C, a strain lacking c-di-AMP is unable to grow at high potassium concentrations because of the unlimited influx of the ion via KtrCD. The potassium exporters are inactive in the absence of the second messenger. Therefore, growth of this strain in the presence of potassium requires a novel active potassium exporter. Under these conditions, the bacteria acquire mutations affecting the NhaK cation/proton antiporter that result in increased potassium export (4).