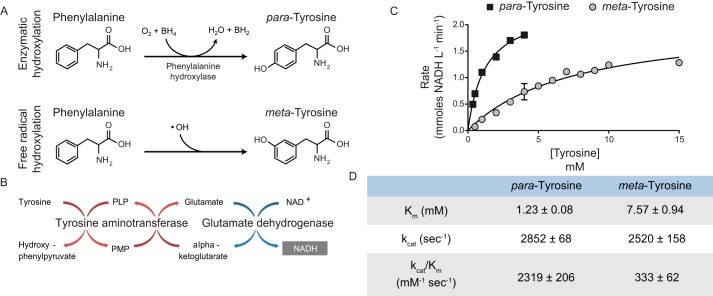
Figure 3.
TATN-1 maintains activity with m-tyrosine as a substrate. A, schematic of the enzymatic (top) and free radical–mediated (bottom) hydroxylation of phenylalanine that results in the formation of the tyrosine isomers para- and meta-tyrosine. B, diagram of the tyrosine aminotransferase/glutamate dehydrogenase coupled reaction used to measure TATN-1 kinetics. In this reaction, glutamate, which is a product of the tyrosine aminotransferase reaction, is converted back to α-ketoglutarate by glutamate dehydrogenase. NAD+ serves as a cofactor for the glutamate dehydrogenase reaction and is reduced to NADH, which absorbs light at a wavelength of 340 nm and can be measured by spectrophotometry. C, Michaelis–Menten plots for TATN-1 activity with para- and meta-tyrosine as substrates. The initial velocities of the reaction at each of the indicated substrate concentrations was measured in triplicate. Mean rates of enzymatic activity and S.E. (error bar) are shown for both tyrosine isomers. D, the calculated kinetic parameters for TATN-1 with either p- or m-tyrosine as a substrate. Ranges represent S.E.