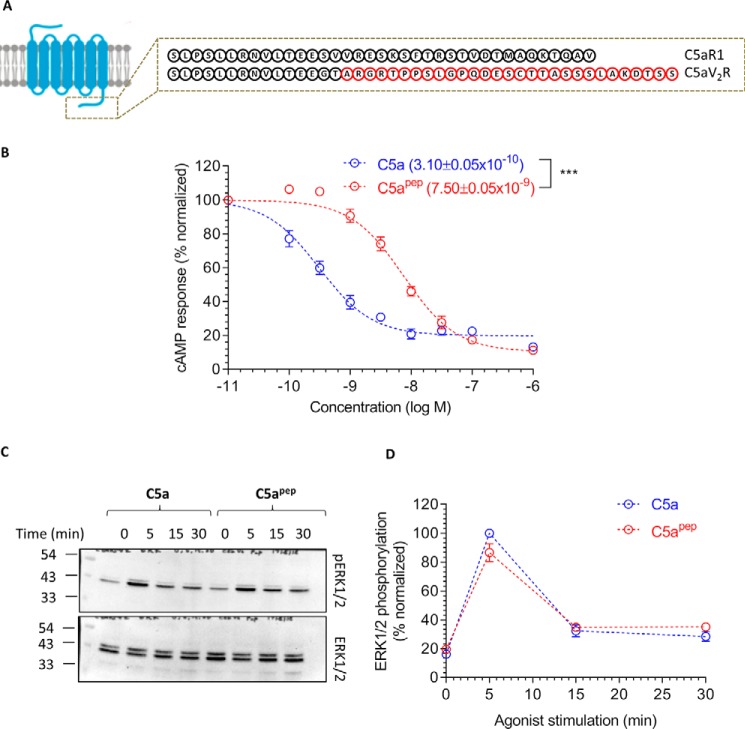
Figure 6.
C5apep exhibits full-agonist efficacy for cAMP response and ERK1/2 phosphorylation for a chimeric C5aR1, C5a-V2R. A, schematic representation of the C terminus of C5aR1 and a chimeric construct harboring the C terminus of AVPR2 (V2R), referred to as C5a-V2R. V2R tail in the chimeric construct is highlighted in red. B, C5apep behaves as a full agonist in GloSensor-based cAMP assay. HEK-293 cells expressing C5a-V2R were transfected with F22 plasmids. 24 h post-transfection, the cells were stimulated with the indicated concentrations of C5a and C5apep followed by recording of bioluminescence readout. The data represent averages ± S.E. of three independent experiments, each carried out in duplicate, and the EC50 values of C5a and C5apep were analyzed using unpaired t test. ***, p < 0.001. C, C5apep induces robust phosphorylation of ERK1/2 MAP kinase at levels similar to C5a. HEK-293 cells expressing C5a-V2R were stimulated with respective ligands (C5a, 1 μm; C5apep, 10 μm) for the indicated time points followed by measurement of ERK1/2 phosphorylation using Western blotting. D, densitometry-based quantification of ERK1/2 phosphorylation data presented in C (average ± S.E.) of five independent experiments.