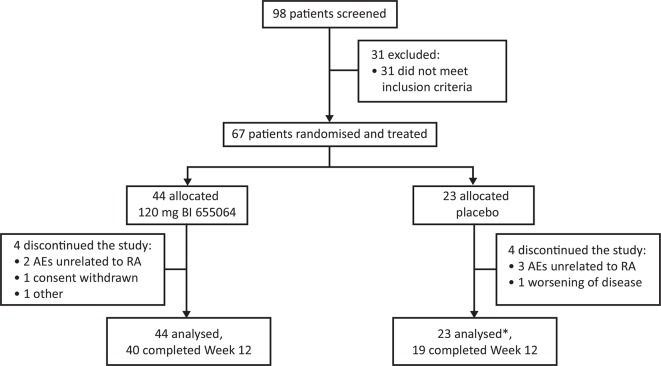
Figure 1.
Patient disposition. Disposition of the study patients treated with subcutaneous 120 mg BI 655064 or placebo administered once weekly for 12 weeks. Discontinuations due to AEs unrelated to RA were a fatal cerebral haemorrhage and one case of iron deficiency in the BI 655064 group and one case of pleural effusion, one case of elevated ALT and AST and one case of nasopharyngitis in the placebo group.*One patient excluded from full analysis set due to insufficient efficacy data; all 67 patients treated were included in the safety analysis set.AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; RA, rheumatoid arthritis.