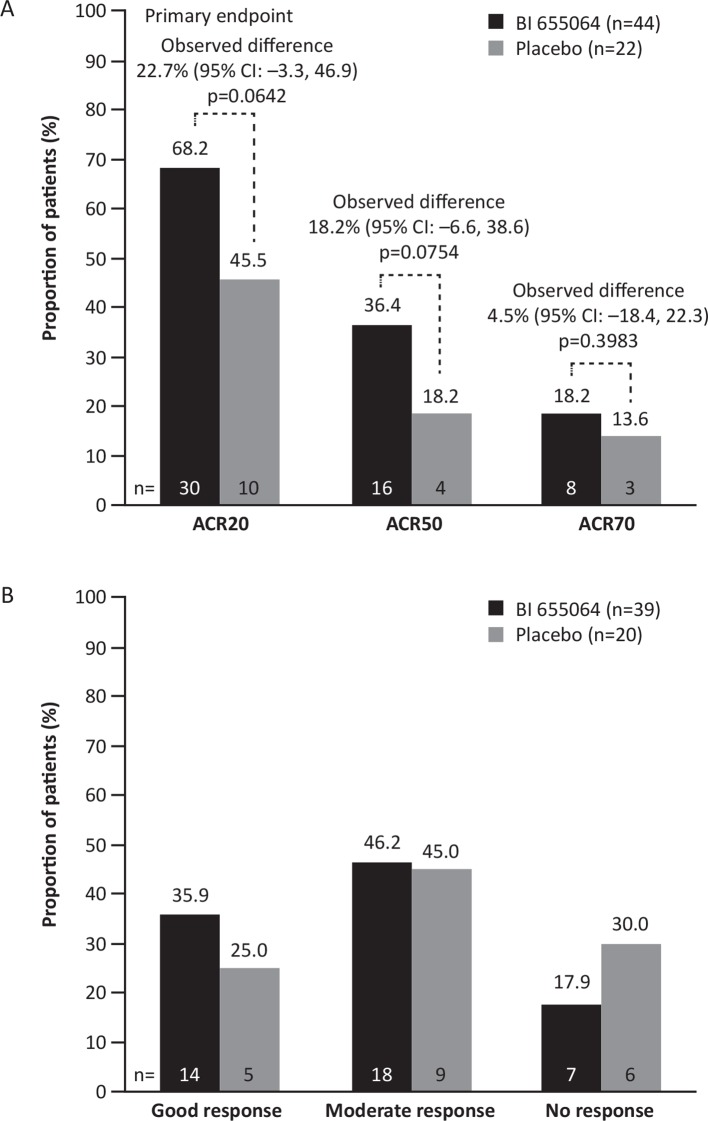
Figure 2.
Treatment response at week 12. Responses were defined according to (A) ACR20/50/70 improvement criteria (FAS, non-responder imputation) and (B) EULAR response (DAS28-CRP; FAS, observed). The primary endpoint (ACR20 at week 12) was evaluated with a Bayesian approach. ACR20/50/70, American College of Rheumatology 20/50/70% improvement criteria; DAS28-CRP, Disease Activity score in 28 joints based on C-reactive protein; EULAR, European League Against Rheumatism; FAS, full analysis set.