Abstract
Primary aging is the progressive decline in health and fitness and depends on metabolic rate and oxidative stress. Untoward changes in body composition and metabolic function characterize secondary aging. We hypothesize that both exercise and calorie restriction improve secondary aging, but only calorie restriction improves primary. However, calorie restriction followed with exercise is a superior strategy for overall healthy aging.
Keywords: exercise, calorie restriction, aging, metabolism, body composition
Summary for Table of Contents:
Calorie restriction yields greater improvements than exercise in determinants of lifespan by attenuating oxidative damage.
INTRODUCTION
Human aging describes the progressive decline in the body’s ability to maintain physiological homeostasis, ultimately causing death. Aging results from a complex set of lifespan-associated declines in molecular, cellular, tissue, and organ fidelity. This set of changes has been categorized into “Seven Pillars of Aging” and includes macromolecular damage, epigenetics, inflammation, adaptation to stress, proteostasis, stem cells and regeneration, and metabolism (1). With advanced biological age, homeostasis of these processes deteriorates independently and synergistically. Despite the inevitable advancement of biological age, the deterioration of physiological homeostasis is variable.
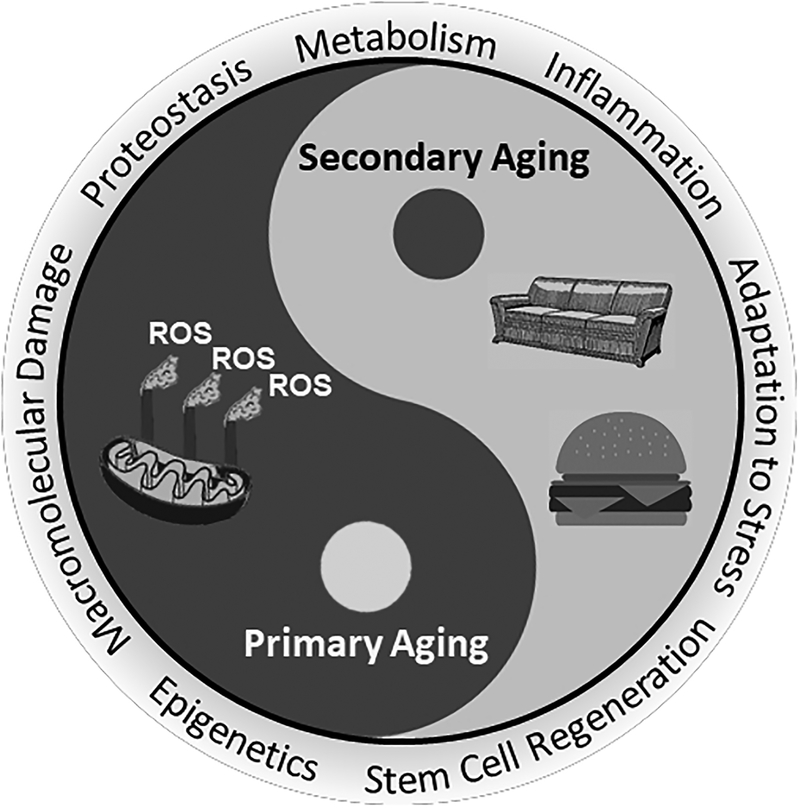
The underlying causes of these physiological deteriorations remain controversial and there are over 300 theories of mammalian aging. The most prominent and most studied causes of aging can be partitioned into either primary or secondary aging. Primary aging describes the effect of the inevitable, yet variable, progression of physiological decline, whereas secondary aging describes the effect of external factors such as diet and physical activity that can accelerate or decelerate the aging process. Both primary and secondary aging factors influence each other in a cyclic, bi-directional fashion, which can either potentiate or attenuate effects on physiological homeostasis (Figure 1).
Figure 1:
Schematic of the interplay between primary and secondary aging. Primary aging is an unavoidable consequence of living stemming from the accumulation of macromolecular damage during lifespan progression. These processes are cyclic, and thus continual oxidative damage favors further generation of ROS ultimately impairing homeostasis and function of cells and tissues, and hence defines primary aging. On the other hand, secondary aging results from external influences originating throughout the lifespan as the result of non-communicable diseases, environmental exposures, and social behaviors such as overeating or low physical activity. These two intertwined factors encompass the seven unique pillars of aging
Primary aging relates to the age-associated decline in physiological homeostasis to high energy expenditure and increased oxidative stress and is based on the Rate of Living Theory. This theory postulates that organisms with higher mass-specific energy expenditures have shorter lifespans (2). The Rate of Living Theory has received mechanistic support by the Free Radical Theory of Aging (3). The Free Radical Theory of Aging assumes that approximately 1%-3% of oxygen consumed by the electron transport system leads to generation of the free radical, superoxide O2−●, by the one-electron reduction of O2. Therefore, with higher energy expenditure more reactive oxygen species (ROS) are generated, which can lead to more oxidative damage to mitochondrial DNA, proteins, and lipids that are essential for normal cellular function (3, 4). These processes are cyclic, and thus continual oxidative damage favors further generation of ROS ultimately impairing homeostasis and function of cells and tissues, and hence defines primary aging (5).
Secondary aging results from external influences originating throughout the lifespan as the result of non-communicable diseases, environmental exposures, and social behaviors such as overeating or low physical activity (6). Overeating increases fat mass, and together with insufficient physical activity results in declines in skeletal muscle strength, mass, and quality. This body composition phenotype leads to the development of metabolic dysfunction, such as impaired clearance and storage of postprandial blood glucose and lipids. The consequent hyperglycemia, hyperinsulinemia, insulin resistance, and hyperlipidemia produces a disproportionate accumulation of fat in both visceral and ectopic depots, increasing risk for type 2 diabetes and cardiovascular diseases (7, 8). Aside from the increased mortality directly associated with these diseases, determinants of secondary aging also impair mitochondrial function and increase oxidative stress, and thereby contribute to primary aging.
In efforts to extend healthspan and lifespan in humans, science has focused on investigating the independent and synergistic effects of energy restriction (termed calorie restriction) and physical activity (exercise) modifications as strategies to mitigate both intrinsic and extrinsic factors that accelerate primary and secondary aging, respectively. This review outlines key studies — particularly human studies performed by the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE) team — that examined how calorie restriction, exercise, or a combination, can improve overall health. Based on the available literature, we hypothesize that calorie restriction yields more robust and consistent improvements in mechanisms associated with healthspan and lifespan because of its independent effects on attenuating the rate of living and oxidative damage (primary aging), as well as reducing adiposity (secondary aging).
CALORIE RESTRICTION
Calorie restriction (CR) with adequate nutrition is the most studied, experimental, non-genetic, non-pharmacological intervention used to extend healthspan and lifespan. CR is defined as a sustained reduction of habitual energy intake, typically by 20%-50%, with maintenance of adequate micronutrient intake (9). The review will focus on studies of CR (10%-30%) in the absence of malnutrition (e.g., extreme CR or chronic eating disorders such as anorexia nervosa). In animal studies, CR prolongs median and maximal lifespan up to 50%, and prevents or delays the onset of many chronic diseases, such as obesity, type 2 diabetes, and cancer (10).
The first randomized controlled trials (RCT) of CR in healthy, non-obese humans were the CALERIE-1 and CALERIE-2 trials. In CALERIE-1, different modalities to induce CR were tested over 6–12 months, which included calorie restriction, calorie restriction plus exercise, and a very-low-calorie diet to induce weight loss as fast as possible. CALERIE-2 subsequently tested the most effective intervention on aging parameters, which was calorie restriction without exercise, over 24 months (11, 12). Evidence on the effect of longer CR on human aging is derived from observational studies in members of the Calorie Restriction Society, who follow a regimen of self-imposed CR with Optimal Nutrition (CRON) with the belief that this dietary lifestyle will prolong lifespan. Individuals adherent to a CRON-diet (referred to as “CRONIES”) for 3–15 years have been reported to voluntarily restrict their caloric intake by approximately 50% as compared to a group of individuals (matched for age, sex, and socioeconomic status) consuming a typical Western diet and not following CR (13).
Primary Aging.
CR mitigates central mechanisms involved in primary aging in both short-term and long-term trials by reducing energy flux and oxidative stress, as well as improving mitochondrial function. First, energy expenditure measured over 24 hours, during sleep, or at rest decreased by 6% more than what was expected from a reduced body mass — otherwise known as a ‘metabolic adaptation’ — after 6 and 12 months (11, 12, 14). The decrease in resting metabolic rate was diminished at 24 months due to reduced adherence; however, the metabolic adaptation observed during sleep was still present at 24 months in subjects who maintained more than 5 kg of weight loss (14). Second, moderate CR (~15%-25%) achieved in both CALERIE-1 and CALERIE-2 reduced oxidative stress as indicated by reduced DNA-damage, superoxide dismutase (SOD)-activity, and F2-isoprostane concentrations in whole blood cells and in plasma, respectively (10, 14–16). These improvements were likely mediated by CR-induced improvements in mitochondrial capacity by increasing mitochondrial DNA (mtDNA) content or inducing mitochondrial biogenesis (15). Furthermore, CR induced a more coupled mitochondrial phenotype (P:O-ratio) (17); however, these effects were only observed in subjects with higher coupling rates before initiating CR.
The results of our CALERIE trials are supported by studies on the CRONIES. While no measurements of metabolic rate have been performed among the CRONIES, skeletal muscle biopsies revealed that PI3K/AKT/FOXO and AMP-activated protein kinase (AMPK)/Sirtuin (SIRT) pathways resemble younger-aged individuals (18). For instance, FOXO activation is up-regulated in several anti-aging genes and proteins, including the antioxidant enzyme SOD2, the DNA repair transcript DDB1, and autophagy genes beclin-1 and LC3 (18, 19). Transcript and protein expression levels of stress-induced cytosolic chaperones HSP70 and GRP78 were also significantly higher in skeletal muscle after CR (19). To our knowledge, this is the first set of data showing that long-term CR in humans upregulates the HSF/HSP pathway and downregulates the activity of the insulin/IGF pathway, which have been shown to play key roles in promoting health and longevity in several experimental model organisms (20, 21).
Secondary Aging.
CR also mitigates mechanisms involved in secondary aging. Specifically, 25% CR significantly reduced body weight (-10%) and fat mass (-24%) after 6 months, and these losses were maintained after 2 years of sustained CR (11, 12, 22). Sustained CR also reduced ectopic lipid accumulation (23), lipidemia (24), systemic inflammation (25), and blood pressure (24), as well as increased insulin sensitivity (23, 24) and pancreatic β-cell function (23). Collectively, these improvements lower the 10-year risk for cardiovascular mortality (24, 25) and risk for developing type 2 diabetes. In support of the longer-term benefit and feasibility of CR, CRONIES had remarkably low risk factors for secondary aging (e.g., BMI 19.7±1.8 kg/m2), which are comparable to age-, sex-, and body fat-matched endurance runners (13, 26, 27).
Despite these beneficial effects of CR, sustained CR also has some drawbacks. CR results in the loss of fat-free (muscle) mass and a decline in concentric isokinetic and isometric strength, and cardiorespiratory fitness (28, 29). When expressed per kilogram of body mass, however, maximal aerobic capacity (VO2peak/VO2max) was maintained or increased with CR and suggests that a reduced body weight may preserve or even improve physical functioning. These effects may be dependent on sex (strength and function increased more in males than females) or on ad libitum physical activity levels (males maintained physical activity, whereas physical activity decreased in females).
The reduction in energy expenditure observed with CR while beneficial towards primary aging, may favor a positive energy balance (e.g., weight gain) if CR is temporarily discontinued and is therefore a risk factor for secondary aging (30). Reduced fat-free mass and hence muscle mass, may also increase the risk for frailty in older populations. Indeed, reductions in bone mineral density have been observed with CR in older adults (31). Whether bone loss is larger than expected on the basis of weight loss is important to assess on an individualized basis especially among individuals whose bone mineral density may be compromised, such as elderly individuals (31).
EXERCISE
Traditionally, exercise is defined as physical activity that is planned, structured, repetitive, and is purposefully conducted and can range from recreational activities to competitive sports. Although resistance training has profound effects on both muscle and cardiometabolic health, the majority of studies examining the ability of lifelong exercise to extend the healthspan or lifespan in pre-clinical and clinical models have focused on endurance (aerobic)-based physical activities. For the purpose of this review, exercise that is purposely conducted to meet physical activity recommendations, though not to the extent of extreme training (e.g., marathons) is discussed. Despite much interest, researchers have unsuccessfully demonstrated that lifelong physical activity or exercise can extend maximal lifespan by slowing primary aging in well-controlled longitudinal studies in humans. However, maintaining physical activity at this level increases the disability-free mean/median lifespans. Specifically, lifelong exercise is associated with reductions in the risk of premature CVD mortality and related risk factors (e.g., hypertension, hypercholesterolemia, and type 2 diabetes) (32). These improvements share common and independent mechanisms with CR related to primary and secondary aging.
Primary Aging.
Exercise improves physiological functioning (e.g., increases mitochondrial content and oxidative capacity, reduces oxidative stress, and enhances protein quality control) which may attenuate primary aging. To date, it is unclear whether exercise affects metabolic rate after the recovery-phase (24–72h), independent of changes in body composition. Only when combined with caloric restriction, has a reduction in metabolic rate been reported following a six-month intervention (11). The effects of exercise and physical activity on oxidative stress and mitochondrial capacity are better characterized. Seminal work in controlled intervention studies performed in the late 1960s reported that exercise increases mitochondrial abundance and upregulates oxidative enzyme activities, as well as increases oxidative capacity in skeletal muscle (33). In pre-clinical studies from the laboratory of the late John Holloszy, sustained periods of exercise increased the mean/median lifespan in rodents with little evidence that exercise extends maximal lifespan (34). Studies in humans show that regular exercise increases mitochondrial oxidative capacity (35, 36) and partially normalizes mitochondrial oxidative capacity as well as enzyme activities in trained older adults compared to sedentary younger adults (36). Despite these improvements in mitochondrial bioenergetics, regular exercise does not completely ameliorate age-related reductions in mtDNA content which may reflect a true defect in primary aging (37). These mtDNA mutations could reduce the ability of older adults to increase their mtDNA abundance in response to exercise. However, sedentary elderly subjects participating in an aerobic exercise training program increased skeletal muscle mitochondrial content and function, which suggests that overall exercise-induced mitochondrial biogenesis is preserved with age (38).
Exercise acutely increases oxidative stress, which stimulates antioxidant defense mechanisms that reflect a negative feedback. Excess acute exercise or insufficient antioxidant responses, exercise training carries the risk of increasing rather than decreasing oxidative stress. The induction of a sufficient response to counteract exercise-induced stress is referred to as adaptive homeostasis (39). Through this process, an individual can cope with oxidative stress by repairing damage by free radicals to maintain homeostasis. It should be noted, however, that the balance between exercise-induced energy flux and levels of antioxidants, reactive oxygen species, and reactive nitrogen species may be differentially impacted by disease state, intensity, duration, gender, age, and level of fitness (40). While adaptive homeostasis is crucial for the training effect, it may be deleterious when increased chronically (overtraining) or in the presence of insufficient antioxidant capacity (intrinsic or dietary).
Secondary Aging.
Insufficient levels of moderate to vigorous physical activity are routinely associated with reductions in average lifespan, which is indicative of accelerated secondary aging (41). Lifelong exercise in humans increases the disability-free and mean/median lifespans likely due to reductions in secondary aging and premature mortality. Masters athletes who have maintained high levels of lifelong exercise have reduced risk of premature mortality and increased mean/median lifespans compared to sedentary controls likely due to reductions in secondary aging parameters including adiposity, insulin resistance and hyperlipidemia (42). However, due to the healthier lifestyle that Masters athletes tend to practice including healthy eating habits, reductions in premature mortality and elevations in their mean/median lifespan cannot be exclusively attributed to exercise.
Regular exercise is important for reducing the risk of premature mortality in part by counteracting contributors to secondary aging. For example, regular exercise reduces risk of developing cardiometabolic diseases by reducing abdominal visceral fat (36, 43), dyslipidemia (44), and inflammation (26), while also improving insulin sensitivity (26, 36). Age-related declines in skeletal muscle quality and function are often attributed to declines in physical activity and thus likely contribute to the deleterious impact of secondary aging on overall muscle health. Collectively, these improvements in muscle health serve as an excellent countermeasure against the development of physical frailty and disability. In addition, regular exercise reduces the risk of physical frailty and disability by increasing cardiorespiratory fitness (i.e., VO2max) (35–37), and skeletal muscle mass, strength, and quality (35). Indeed, lifelong exercise has been shown to help maintain cardiorespiratory fitness values that are well above the critical threshold for developing frailty (~5 METS or 17.5 ml/kg/min) (37). In addition, a 1-MET increase in VO2max is associated with ~15% reduction in all-cause mortality (37).
The role of exercise in reducing adiposity, particularly visceral adiposity, is still not fully elucidated. For instance, while a systematic review concluded reductions in visceral adiposity occur in a dose-dependent manner (45), a more recent RCT in middle-aged men and women with obesity revealed that there were no differences between exercise doses (volume and intensity) in the reduction of visceral fat (43). To add further complexity, energy expenditures may need to be greater than 500–600 kilocalories per exercise session to achieve exercise-induced weight and visceral fat loss, which is well above the recommended levels of physical activity (46). Furthermore, the inability of exercise to consistently reduce visceral fat stores could be attributed to the following factors: 1) low exercise doses may be inadequate for inducing a negative energy balance; 2) exercise may induce a compensatory increase in energy intake; 3) poor adherence to exercise prescriptions; or 4) their synergistic effects (47).
An important consideration for use of exercise as part of anti-aging interventions is the implementation of appropriate exercise dosing. Exercise doses above the general exercise recommendations for overall health result in compensatory mechanisms (e.g., increased energy intake or decreased spontaneous physical activity) that can counteract weight loss success (47). As a result, exercise may be a better strategy for weight maintenance after initial weight loss rather than an immediate first-line approach. Future studies are warranted to determine optimal exercise dosing strategies that counteract secondary aging by inducing a negative energy balance that are both sustainable and attainable in the long-term, which could lead to reductions in premature mortality and improvements in overall quality of life.
CALORIE RESTRICTION COMBINED WITH EXERCISE
Well-controlled studies from our group and others have compared exercise versus CR on attenuating factors associated with primary and secondary aging. The 12-month CALERIE-1 trial in overweight adults found that both exercise and CR resulted in similar reductions in energy expenditure (48), as well as reductions in markers of DNA and RNA damage (49). Exercise with CR compared with CR alone resulted in no increased reductions in body weight and adiposity, and improvements in insulin sensitivity and glucose tolerance were comparable (48). This work was replicated by our team over a 6-month period with the added benefit of improvements in aerobic capacity (i.e., VO2max) with exercise (28). In our CALERIE-1 and 2 cohorts of overweight adults (25–50 years), we reported that a combination of CR and exercise showed similar improvements in cardiovascular risk factors including blood pressure, lipidemia, heart rate variability compared to CR alone (24, 25). These benefits may be more pronounced in aging populations given that combining exercise and CR has better outcomes than CR alone.
In older adults, CR and exercise together appear to have an additive effect on glucose homeostasis compared to CR alone (45–65 years), even after matching for energy deficit (50). Indeed, CR with the inclusion of exercise has been shown to preserve not only fat-free mass (i.e., lean muscle mass, bone density), but also enhance aerobic capacity and increase strength. Therefore, incorporating both CR and exercise might be most relevant in elderly populations who are at risk for frailty and falls.
It must be acknowledged that there are some shared, and unique, limitations to both CR and exercise; thus, determining the most suitable approach for an individual requires an understanding of the benefits and caveats to both. The outcomes of either approach equally depend on individual adherence. In addition, the risk of weight regain exists in response to both interventions. Interestingly, the unique drawbacks of either approach may be partially offset by the other. For example, a limitation specific to CR is the reduction in fat-free mass, skeletal muscle strength, and bone mineral density. In contrast, exercise stimulates muscle protein synthesis and may counteract declines in skeletal muscle mass, strength, and bone mineral density. A limitation specific to exercise is the increase in oxidative stress which may negatively influence health in high doses. In contrast, CR reduces oxidative stress markers. Weighing the drawbacks of either approach, concurrent implementation of CR and exercise in a complementary and sustainable fashion may yield the greatest benefit.
CONCLUSION
To test our hypothesis that CR is superior to exercise as an anti-aging intervention, we evaluated the effects of CR and exercise on determinants of primary and secondary aging through critical review of the literature. Whereas exercise is more important for maintaining overall fitness and strength, we recommend initial weight loss through CR and maintenance of this weight loss with increased exercise in a dose-dependent manner. Appropriate application of either intervention should take an individual’s age and health status into account and whether it is sustainable. Interventions will only be as effective as the overall compliance of the individual. Long-term sustainability should be assessed, as compliance determines the effectiveness of an intervention. Young and middle-aged adults may receive the most benefit from CR as a lifespan extension strategy, compared to older adults. The modern obesogeneic environment is another factor that should be considered. Given the widespread accessibility of calorically-dense foods, CR is the most efficacious lifestyle intervention for those individuals that have clinically relevant amounts of adiposity to lose. Alternatively, exercise may be more appropriate for elderly individuals, as it preserves lean muscle mass, improves strength, and decreases fragility. Overall, more RCTs combining both CR and exercise are needed across multiple time points throughout life and in various metabolic disease states to ensure the most adequate weight loss recommendations are prescribed. In addition, future efforts should aim to identify primary physiological and behavioral determinants of long-term adherence to healthier lifestyle choices across the lifespan.
To conclude, we found more evidence to support that CR, but not exercise, reduces metabolic rate and lowers oxidative stress in humans, and thus seems more effective for attenuating determinants of primary aging (Figure 2). CR may be a superior lifestyle modification strategy to improve healthspan and promote longevity via reductions in metabolic rate and oxidative stress, whereas clinical trials of exercise do not consistently demonstrate these improvements. In regards to secondary aging, we found that both CR and exercise beneficially reduce adiposity. Taken together, the current state of the literature suggests that CR leads to more robust and sustained improvements in primary and secondary aging factors than exercise. Thus, CR may be a superior lifestyle modification strategy to improve healthspan and promote longevity via reductions in metabolic rate and oxidative stress, whereas clinical trials of exercise do not consistently demonstrate these improvements. More long-term studies are needed to fully elucidate the synergistic and separate effects of either lifestyle modification approaches on human aging and to identify strategies to predict adherence.
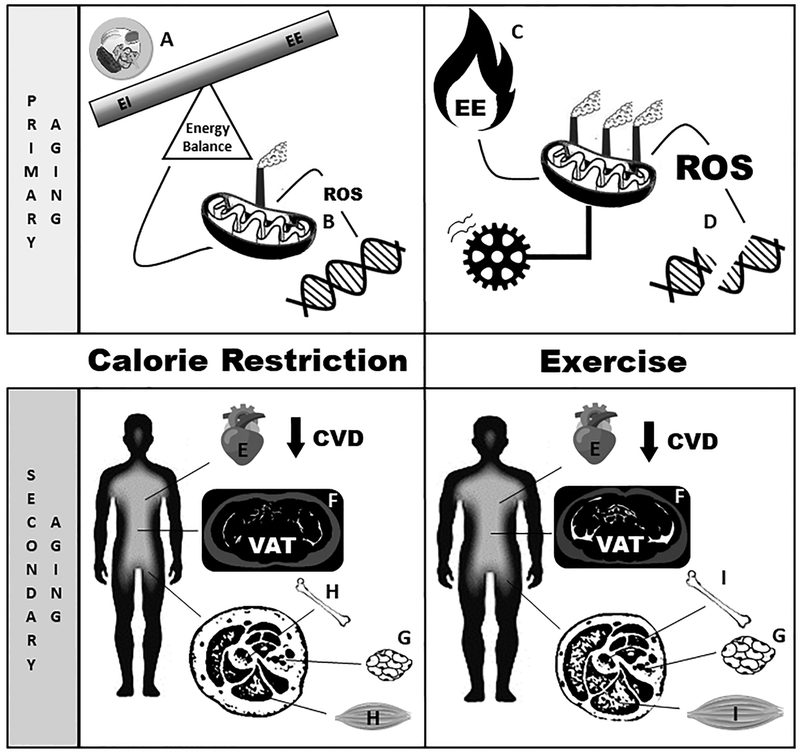
Figure 2:
Schematic diagram representing the effect of calorie restriction (CR) and exercise on components of primary and secondary aging. Primary aging is the progressive physiological decline, which depends on in body functioning and depends on ones metabolic rate and oxidative stress. A.) CR exerts its effect on primary aging via reductions in energy intake and subsequently less flux of macronutrients through the mitochondria. B.) In turn, mitochondria release less reactive oxygen species (ROS) inducing a state of less oxidative damage to DNA. C.) On the other hand, exercise increases energy expenditure, which increases energy flux through the mitochondria and leads to higher generation of ROS and oxidative damage (D.). Secondary aging is defined as untoward change in body composition and metabolic function. E.) In regard to secondary aging, CR has been shown to improve biomarkers associated with cardiovascular disease (CVD) and reductions in adiposity. These reductions are primarily through decreases in both F.) visceral adipose tissue (VAT) and G.) subcutaneous adipose tissue. H.) However, CR also leads to loss of fat-free mass (muscle and bone). Likewise, exercise has shown to improve biomarkers associated with CVD (E.) and reduces adiposity (F., G.), albeit not to the extent of CR, but maintain fat-free mass (I.). CVD=cardiovascular disease, EE=energy expenditure, EI=energy intake, ROS=reactive oxygen species, VAT=visceral adipose tissue.
Key Points.
Human life expectancy has increased over past centuries due to improved medical care. However, lifestyle habits including poor diet quality, overeating, and physical inactivity have attenuated this increase.
The progressive decline in physiological homeostasis and physical performance and fitness with increasing age that is driven by energy flux is defined as primary aging. Secondary aging, or healthspan, is defined as any untoward change in body composition and metabolic function that reduce health and lifespan.
To improve human lifespan, successful anti-aging interventions need to counteract both primary and secondary causes of aging.
Calorie restriction and exercise both extend healthspan; however, calorie restriction is superior to exercise because of the additional attenuation of primary aging.
ACKNOWLEDGMENTS
This work was funded by the NIH (R01 AG029914 and U01 AG020478) and supported in part by P30DK072476 (Pennington/Louisiana NORC). We are indebted to the commitment of the study participants who invested over 2 years of their lives to participate in this clinical trial. The CALERIE phase 2 trial (NCT00427193) and the ancillary study (NCT02695511) are registered as clinical trials at http://clinicaltrials.gov/. This review is supported in part by 1R21-AG058181–01A1 (B.A.I.) and the NIDDK sponsored Ruth L. Kirschstein National Research Service T32 Research Training Grant (T32-DK064584) (K.L.M.).
Funding: This work was funded by the NIH (R01 AG029914 and U01 AG020478) and supported in part by P30DK072476 (Pennington/Louisiana NORC).
Footnotes
Conflict of Interest Disclosure: The authors have nothing to disclose.
REFERENCES
- 1.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacher GA, Duffy PH. Genetic relation of life span to metabolic rate for inbred mouse strains and their hybrids. Fed Proc. 1979;38(2):184–8. [PubMed] [Google Scholar]
- 3.Harman D Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. [DOI] [PubMed] [Google Scholar]
- 4.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holloszy JO. The biology of aging. Mayo Clin Proc. 2000;75 Suppl:S3–8; discussion S-9. [PubMed] [Google Scholar]
- 7.Boren J, Taskinen MR, Olofsson SO, Levin M. Ectopic lipid storage and insulin resistance: a harmful relationship. J Intern Med. 2013;274(1):25–40. [DOI] [PubMed] [Google Scholar]
- 8.Liu HH, Li JJ. Aging and dyslipidemia: a review of potential mechanisms. Ageing Res Rev. 2015;19:43–52. [DOI] [PubMed] [Google Scholar]
- 9.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42(8):709–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78(3):361–9. [DOI] [PubMed] [Google Scholar]
- 11.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravussin E, Redman LM, Rochon J, et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci. 2015;70(9):1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101(17):6659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018;27(4):805–15 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Civitarese AE, Carling S, Heilbronn LK, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4(3):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Il’yasova D, Fontana L, Bhapkar M, et al. Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: The CALERIE 2 randomized clinical trial. Aging Cell. 2018;17(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparks LM, Redman LM, Conley KE, et al. Effects of 12 Months of Caloric Restriction on Muscle Mitochondrial Function in Healthy Individuals. J Clin Endocrinol Metab. 2017;102(1):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercken EM, Crosby SD, Lamming DW, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12(4):645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Licastro D, Cava E, et al. Long-Term Calorie Restriction Enhances Cellular Quality-Control Processes in Human Skeletal Muscle. Cell Rep. 2016;14(3):422–8. [DOI] [PubMed] [Google Scholar]
- 20.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–5. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama K, Fukumoto K, Murakami T, et al. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516(1–3):53–7. [DOI] [PubMed] [Google Scholar]
- 22.Das SK, Roberts SB, Bhapkar MV, et al. Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. Am J Clin Nutr. 2017;105(4):913–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Most J, Gilmore LA, Smith SR, Han H, Ravussin E, Redman LM. Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am J Physiol Endocrinol Metab. 2018;314(4):E396–E405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefevre M, Redman LM, Heilbronn LK, et al. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203(1):206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr). 2010;32(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47(2):398–402. [DOI] [PubMed] [Google Scholar]
- 28.Larson-Meyer DE, Redman L, Heilbronn LK, Martin CK, Ravussin E. Caloric restriction with or without exercise: the fitness versus fatness debate. Med Sci Sports Exerc. 2010;42(1):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Racette SB, Rochon J, Uhrich ML, et al. Effects of Two Years of Calorie Restriction on Aerobic Capacity and Muscle Strength. Med Sci Sports Exerc. 2017;49(11):2240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318(8):467–72. [DOI] [PubMed] [Google Scholar]
- 31.Villareal DT, Fontana L, Weiss EP, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166(22):2502–10. [DOI] [PubMed] [Google Scholar]
- 32.Maessen MF, Verbeek AL, Bakker EA, Thompson PD, Hopman MT, Eijsvogels TM. Lifelong Exercise Patterns and Cardiovascular Health. Mayo Clin Proc. 2016;91(6):745–54. [DOI] [PubMed] [Google Scholar]
- 33.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242(9):2278–82. [PubMed] [Google Scholar]
- 34.Holloszy JO. Exercise and longevity: studies on rats. J Gerontol. 1988;43(6):B149–51. [DOI] [PubMed] [Google Scholar]
- 35.Irving BA, Lanza IR, Henderson GC, Rao RR, Spiegelman BM, Nair KS. Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. J Clin Endocrinol Metab. 2015;100(4):1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanza IR, Short DK, Short KR, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57(11):2933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gries KJ, Raue U, Perkins RK, et al. Cardiovascular and Skeletal Muscle Health with Lifelong Exercise. J Appl Physiol. 2018;125(5):1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broskey NT, Greggio C, Boss A, et al. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014;99(5):1852–61. [DOI] [PubMed] [Google Scholar]
- 39.Pomatto LCD, Davies KJA. Adaptive homeostasis and the free radical theory of ageing. Free Radic Biol Med. 2018;124:420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkler M, Lichtenberg D, Pinchuk I. The relationship between oxidative stress and exercise. J Basic Clin Physiol Pharmacol. 2014;25(1):1–11. [DOI] [PubMed] [Google Scholar]
- 41.Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol (1985). 2011;111(5):1497–504. [DOI] [PubMed] [Google Scholar]
- 42.Kontro TK, Sarna S, Kaprio J, Kujala UM. Mortality and health-related habits in 900 Finnish former elite athletes and their brothers. Br J Sports Med. 2018;52(2):89–95. [DOI] [PubMed] [Google Scholar]
- 43.Cowan TE, Brennan AM, Stotz PJ, Clarke J, Lamarche B, Ross R. Separate Effects of Exercise Amount and Intensity on Adipose Tissue and Skeletal Muscle Mass in Adults with Abdominal Obesity. Obesity (Silver Spring). 2018;26(11):1696–703. [DOI] [PubMed] [Google Scholar]
- 44.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–92. [DOI] [PubMed] [Google Scholar]
- 45.Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond). 2007;31(12):1786–97. [DOI] [PubMed] [Google Scholar]
- 46.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133(2):92–103. [DOI] [PubMed] [Google Scholar]
- 47.Thomas DM, Bouchard C, Church T, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev. 2012;13(10):835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss EP, Racette SB, Villareal DT, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84(5):1033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofer T, Fontana L, Anton SD, et al. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. Rejuvenation Res. 2008;11(4):793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss EP, Albert SG, Reeds DN, et al. Calorie Restriction and Matched Weight Loss From Exercise: Independent and Additive Effects on Glucoregulation and the Incretin System in Overweight Women and Men. Diabetes Care. 2015;38(7):1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]