Abstract
Respiratory motor neuron death arises from multiple neurodegenerative and traumatic neuromuscular disorders. Despite motor neuron death, compensatory mechanisms minimize its functional impact by harnessing intrinsic mechanisms of compensatory respiratory plasticity. However, the capacity for compensation eventually reaches limits and pathology ensues. Initially, challenges to the system such as increased metabolic demand reveal sub-clinical pathology. With greater motor neuron loss, the eventual result is de-compensation, ventilatory failure, ventilator dependence and then death. In this brief review, we discuss recent advances in our understanding of mechanisms giving rise to compensatory respiratory plasticity in response to respiratory motor neuron death including: 1) increased central respiratory drive, 2) plasticity in synapses on spared phrenic motor neurons, 3) enhanced neuromuscular transmission and 4) shifts in respiratory muscle utilization from more affected to less affected motor pools. Some of these compensatory mechanisms may prolong breathing function, but hasten the demise of surviving motor neurons. Improved understanding of these mechanisms and their impact on survival of spared motor neurons will guide future efforts to develop therapeutic interventions that preserve respiratory function with neuromuscular injury/disease.
1. INTRODUCTION
Multiple neuromuscular disorders arising from traumatic, infectious, autoimmune, neurotoxic or genetic neuromuscular conditions induce loss of motor neurons (i.e. α-motor neurons innervating and providing cholinergic input to motor end plate) at different time-scales and severities. Motor neuron death during neurodegenerative diseases versus advanced age occur with very different time-scales; for example, polio kills motor neurons rapidly (1), amyotrophic lateral sclerosis (ALS) kills motor neurons over years, and aging (or post-polio syndrome) causes motor neuron loss over decades. At the onset of neurodegenerative disease, motor deficits induced by motor neuron death can be difficult to detect since the degeneration is incremental, and compensatory plasticity preserves function to a considerable extent. However, once compensatory plasticity reaches its limits with advancing pathology, challenges to the respiratory control system are aggravated, leading to respiratory failure, ventilator dependence, life-threatening lung infections and death (2). Trauma causes the quickest functional decline, detectable immediately after the initial impact but with gradual alleviation or exacerbation of functional deficits with time. Despite the importance of respiratory motor neurons to breathing, we know little concerning compensatory mechanisms in respiratory control during neuromuscular clinical disorders, the factors limiting compensation, and physiological costs associated with employing each strategy.
Compensatory plasticity is based on two main principles: i) utilization of remaining functional reserve, and ii) regeneration of lost force generating capacity. For example, increased central drive, strengthening of existing synapses and accessory muscle recruitment facilitates the use of remaining functional reserve of the respiratory system, which normally utilizes only about 20% of its capacity during resting breathing (3). Reinnervation of denervated muscle fibers via motor neuron end-terminal sprouting with motor neuron death and re-innervation of motor neurons following loss of descending medullary input in high cervical spinal cord injury exemplify regeneration of lost force generating capacity.
The goal of the present review is to emphasize common mechanisms of compensatory plasticity preserving ventilatory function across different neuromuscular disorders involving respiratory motor neuron death. Here, we elaborate on the conceptual framework initially described by Johnson and Mitchell (2013) to improve our understanding of compensatory plasticity via the unique perspectives and approaches focused on different diseases (e.g. ALS, spinal cord injury, post-polio syndrome).
2. RESPIRATORY DEFICITS WITH MOTOR NEURON DEATH
2.1. ALS
ALS is a neurodegenerative disease characterized by progressive and preferential loss of motor neurons, with attendant respiratory and limb muscle paralysis. ALS is caused by genetic or sporadic (e.g. β-methylamino-L-alanine-induced) causes often associated with familial or environmental risk factors (4–6). Patients with ALS typically exhibit progressive respiratory muscle weakness, as evident by reduced maximum inspiratory and expiratory pressures, and sniff nasal or trans-diaphragmatic pressures. Eventually, hypoventilation is observed especially during sleep (7–12). The ability to generate respiratory-related behaviors such as coughing and swallowing is also impaired in ALS, leading to impaired airway defense and risk of respiratory infections (8, 13, 14).
Genetic rodent models of ALS have been developed to mimic aspects of human ALS pathology. As one example, rat and mouse models overexpressing superoxide dismutase 1 (SOD1) with specific gene mutations known to elicit familial ALS in humans (15) exhibit progressive motor neuron death, limb and respiratory muscle paralysis, and eventual death (16–19). In the SOD1G93A rat model, respiratory motor neuron cell death at disease end-stage mimics the human condition (~75% phrenic, ~60% intercostal motor neuron loss; (20, 21). At disease end-stage, spontaneous phrenic nerve activity and evoked compound diaphragm action potentials are blunted (16, 20). On the other hand, spontaneous diaphragm EMG activity during near-maximal reflex activation remains unchanged (21). Nevertheless, trans-diaphragmatic pressure and the esophageal-to-gastric pressure ratio are decreased, suggesting reduced diaphragm muscle contributions to breathing. Surprisingly, ventilatory capacity is unaffected until very late in the disease progression (20, 22). Ventilatory capacity of SOD1G93A mice is preserved even 2 days before overt ventilatory failure (23). Similarly, SOD1G93A rats preserve full ventilatory capacity until a defined end-stage (20, 22).
The respiratory motor system is utilized during ventilatory behaviors (low force) and airway protective reflexes (high force). Fast-twitch muscle fibers are recruited only during near-maximal airway protective behaviors (e.g. sneezing and coughing). Ventilatory measurements are not impacted early in ALS, since motor neurons affected in the pre-symptomatic stage are only recruited during high force generating airway protective behaviors, such as sneezing and coughing; they are not likely recruited during less intense ventilatory behaviors (3, 24, 25). Thus, in presymptomatic ALS stages, the motor neurons innervating the most forceful and fatigable muscle fibers are preferentially affected in rodent ALS models, whereas all motor neuron pools are affected in the symptomatic stages (26, 27). For example, 35% of motor neurons innervating fast-twitch-dominant extensor digitorum longus (EDL) muscle were dead in contrast to 10% loss in slow-twitch-dominant soleus muscle at presymptomatic time-points (day 50) in SOD1G93A mice. At symptomatic stages (day 120), EDL and soleus motor neuron loss were 45% and 35%, respectively. Therefore, muscle force and activation during near-maximal airway protective behaviors (e.g. sneezing and coughing) are likely affected earlier in ALS than ventilatory capacity, a common feature of many respiratory neuromuscular pathologies.
Respiratory insufficiency in human ALS may occur long after respiratory motor neurons and muscles are affected, although this has not been demonstrated directly. Early pathological changes can be detected prior to the functional symptomatic period, suggesting that humans with ALS have been undergoing pathology for some time before symptoms leading to diagnosis. In the SOD1G93A mouse model of ALS, axonal detachment of motor neuron terminals from muscle precedes symptom onset; 47 days vs. 90 days, respectively (28). A significant drop in muscle fiber force (~60%) is reported in pre-symptomatic SOD1G93A mice (26), suggesting secondary pathology. With progressive motor neuron death, muscle fibers are exposed to cycles of denervation and reinnervation, potentially leading to muscle fiber atrophy.
To study the direct implications of respiratory motor neuron death without other complications attendant to disease, a model of respiratory motor neuron death was developed using an engineered toxin (29), Cholera toxin beta subunit (CTB) conjugated to saporin (CTB-Saporin). CTB binds to the GM1 gangliosides present on neurons and is internalized into those neurons. Thus, intramuscular or intrapleural injection of CTB leads to its uptake at the neuromuscular junctions and retrograde transport to the motor neuron somata; this characteristic is often used in motor neuron labeling (30). Saporin inhibits protein translation via inhibition of 28S subunit of ribosomes, leading to cell death (31–33). Thus, when CTB-Saporin is delivered intrapleurally, it is internalized by motor neuron axon terminals and retrogradely delivered to motor neuron somata, where Saporin is cleaved from CTB and released into the cytoplasm where it triggers respiratory motor neuron death. Motor neuron death via intrapleural CTB-Saporin mimics some respiratory impairments observed during end-stage motor neuron disease, such as phrenic and intercostal motor neuron loss, microglial activation, diminished phrenic nerve activity, and relatively preserved tidal volume during maximum chemoreceptor stimulation (29). The rate of motor neuron loss in CTB-Saporin model is quicker than rodent ALS models. Therefore, it is possible that plasticity mechanisms may not cope with accelerated motor neuron loss. In fact, compensatory respiratory plasticity in this model is less robust than that observed in SOD1G93A rats (22, 29). Factors accounting for this difference remain to be investigated.
2.2. SPINAL CORD CONTUSION INJURY
The most common cause of death after cervical spinal cord injury (SCI) is respiratory failure (34). Cervical SCIs often cause phrenic motor neuron death and disrupt descending neural pathways to phrenic and other respiratory motor neurons. Consequently, high-cervical SCIs frequently cause respiratory failure, necessitating mechanical ventilation. Increased mortality due to lung infections occurs in ventilator-dependent patients following SCI (35). On the other hand, mid- and low-cervical SCIs partially spare phrenic motor neurons; nevertheless, there is still potential for respiratory impairment and reliance on ventilatory support depending on the extent of spared neural tissue following injury. Most SCIs are incomplete, sparing neurons and axonal pathways that can undergo compensatory plasticity, spontaneously improving function (36–45). Regardless, peak cough flow, maximal expiratory pressure, and maximal inspiratory pressure are diminished by cervical SCI especially at C5 and above (46). A greater reduction in tidal volume is observed transitioning to during sleep in those with cervical SCI and is associated with higher end-tidal CO2 and lower O2 (47). Central and/or obstructive sleep apnea are highly prevalent following cervical SCI (48, 49).
Injured human spinal cord tissues show extensive gray and white matter damage at the injury epicenter, with sparing around the rim of the spinal cords (50). Motor neuron death (~45% loss) occurs bilaterally in multiple spinal segments starting from 1 level rostral, down to 3 levels caudal to injury epicenter. Loss of motor neurons suggest muscle fiber denervation; together with inactivity, this denervation will lead to severe muscle atrophy after SCI (51, 52).
Rodent models of cervical (contusion) SCI mimic aspects of human SCIs, including impact, hemorrhage, robust demyelination and axonal degeneration that affects multiple spinal segments surrounding the epicenter. As a result of cervical contusion injury, ~50% of phrenic motor neurons may die, leading to Wallerian degeneration and diaphragm motor end plate denervation (53–56). Phrenic motor neuron loss is associated with reduced evoked compound diaphragm muscle action potentials (56), phrenic nerve activity and abnormal ventilatory patterns (42, 57, 58).
While cervical SCI can significantly impact phrenic output or diaphragm activity; tidal volume does not consistently change across different studies following cervical contusions. For example, tidal volume was either recovered or not changed at 2 weeks post-injury (53, 54, 56, 57, 59). On the other hand, some reports demonstrated a long-term decrease in tidal volume (45, 58). It is possible that the level and severity of injury affect the potential for functional compensation and long-term recovery. Therefore, it is critical to provide and compare detailed information about parameters characterizing the contusion impact and the extent of injury by reporting (e.g. applied peak force, impulse, tissue displacement due to impact, hemorrhage, tissue sparing at the epicenter etc.). In addition, there are important considerations in the plethysmographic assessment of respiratory behaviors. First, proper acclimatization and ‘reminder’ protocols should be applied before the first and subsequent measurements, respectively. Second, because dysfunction of thermoregulation is very often observed after SCI (60, 61), body temperatures should be carefully monitored, reported during plethysmographic measurements and tidal volumes should be corrected for actual body temperatures with the Drorbaugh-Fenn Equation (62).
3. MECHANISMS OF COMPENSATORY PLASTICITY
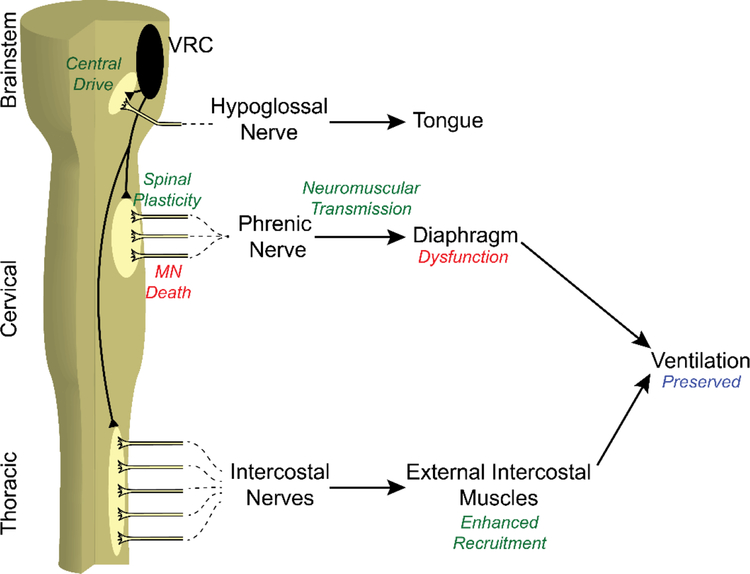
There is no single answer to how the central nervous system compensates to maintain ventilatory capacity in neuromuscular pathologies causing respiratory motor neuron loss. In many cases of disease or trauma, multiple compensatory mechanisms are employed. With a physiological process as critical as breathing, it would be surprising if the affected individual did not use every potential form of compensation at their disposal. Potential mechanisms compensating deficiencies in phrenic motor output include (Figure 1): 1) increased central respiratory drive, 2) spinal synaptic enhancement within the phrenic motor nucleus, 3) enhanced neuromuscular transmission, and 4) shifting respiratory muscle utilization from more affected to less affected motor pools (2, 21, 22).
Figure 1.
Overview of Compensatory Plasticity Mechanisms after Phrenic Motor Neuron Death. Phrenic motor neuron death leads to diaphragm dysfunction, which can be compensated by 1) increased central drive, 2) spinal plasticity at the phrenic nucleus, 3) improved neuromuscular transmission, and 4) enhanced accessory muscle recruitment. These mechanisms preserve ventilatory and airway protective functions of respiratory motor network.
3.1. INCREASED CENTRAL DRIVE
Respiratory motor neurons receive central mono- and poly-synaptic glutamatergic input (central drive) via descending bulbospinal connections from medulla, where respiratory rhythm and pattern are generated. Both respiratory rhythm and pattern are determined via afferent input from chemoreceptors sensing changes in O2, CO2, and pH, as well as mechanoreceptors sensing changes in lung and chest wall volume/pressure. For example, increased CO2 stimulates the central chemoreceptors at the brainstem, increasing tidal volume and frequency. Therefore, chemoreceptor feedback could compensate for challenges such as respiratory motor neuron loss, but only after disease progression is severe enough to cause overt hypoventilation. Animal models of cervical SCI often present decreased tidal volume compensated by increased respiratory frequency (57, 58). This pattern shift may be due to mechanoreceptor feedback and/or neuroplasticity since baseline minute ventilation is not affected by the injury severity reported in these studies.
In rodent ALS models, ventilation is not affected, hypoglossal motor neuron numbers and motor output are maintained until the disease end-stage (20), suggesting that increased central drive is not employed until late in disease progression. In fact, it may not be practical to maintain high levels of glutamate release for long periods, particularly with neurodegenerative diseases. Motor neuron survival depends on proper function of surrounding glial cells, which maintain the extracellular milieu and provide trophic and nutritional support. Demise of interneurons and motor neurons in ALS is a consequence of dysfunction of multiple cell types in the CNS and can be facilitated by endogenous toxic insults such as glutamate excitotoxicity. Glutamate is one of the most important mediators of excitatory synaptic neurotransmission. However, at high concentrations of extracellular glutamate, excess glutamate receptor activation can cause excitotoxic neuron death (63–66). Glutamate uptake at the synaptic cleft by astrocytic glutamate transporters is impaired in ALS (67, 68). In addition, loss of inhibitory spinal interneurons is also implicated to contribute to excitotoxicity (69–71). Thus, multiple mechanisms can contribute to excitotoxic motor neuron death.
If the central respiratory drive is chronically increased and glutamate scavenging mechanisms cannot cope with the demand, chronically increased extracellular glutamate concentrations would likely hasten motor neuron death. Mounting evidence from in vitro and in vivo models suggest that glutamate receptor antagonism can be neuroprotective (65). In fact, riluzole prolongs survival in ALS patients possibly via modulation of glutamatergic system (72, 73). Thus, increased central drive may be detrimental to motor neuron survival ALS; thus, with increased descending drive in late-stage disease, increased central respiratory drive and glutamate release within respiratory motor nuclei is expected to accelerate motor neuron death, leading to the impression that patients “fall off a cliff” as they move towards ventilator dependence.
3.2. SPINAL SYNAPTIC PLASTICITY AT THE PHRENIC MOTOR NUCLEUS
Respiratory spinal networks exhibit considerable plasticity (74–76). For example, activation of Gq and Gs-protein coupled metabotropic receptors elicit phrenic motor facilitation (pLTF), a long-lasting increase in phrenic nerve activity (77–85). pLTF is a form of phrenic motor facilitation induced by moderate or severe cute intermittent hypoxia (mAIH and sAIH, respectively). mAIH-induced pLTF results from a 5HT-2 receptor-dependent mechanism, which requires new BDNF synthesis and TrkB receptor activation (86). In contrast, sAIH-induced pLTF results from an adenosine 2A (A2A) receptor-dependent mechanism (83).
Although central serotonergic neurons degenerate in rodents models (87) and patients with ALS (88), mAIH-induced pLTF is enhanced in late-stage SOD1G93A rats, and the underlying mechanisms remain 5-HT-2 receptor and BDNF-dependent, suggesting that it arises from amplification of the same fundamental mechanism (89). Enhanced pLTF is also observed following intrapleural CTB-Saporin-induced phrenic motor neuron death (90), suggesting that motor neuron death per se is sufficient to elicit certain forms of spinal respiratory motor plasticity. The relevant trigger to this form of plasticity/metaplasticity remains unknown (91). The impact of AIH-induced phrenic motor plasticity on the spontaneous compensatory plasticity remains to be elucidated. Further, it is unknown if this same trigger is sufficient to elicit spontaneous plasticity not linked to AIH. Since ~20% of spared phrenic motor neurons at disease end-stage elicit 55% of normal phrenic nerve activity during maximal chemoreflex activation In end-stage SOD1G93A rats, there must be some form of spontaneous compensatory plasticity that amplifies descending synaptic inputs onto spared phrenic motor neurons (22).
Serotonergic mechanisms of plasticity in the respiratory neural network after cervical spinal injuries are often studied using hemisection injuries. Following cervical hemisection, serotonergic input to motor neurons is often impaired transiently at 2 weeks (92) consistent with mAIH-induced pLTF impairment at 2 weeks post-injury. At 8 weeks post-injury, serotonergic innervation is partially recovered and pLTF is restored. Pharmacological activation of 5HT-1 or 5HT-2 receptors is sufficient to enhance phrenic nerve activity after cervical hemisection injuries (93–95). Repetitive exposure AIH is currently being explored as a minimally invasive therapeutic intervention in rodents and humans. Repetitive exposure to AIH improves respiratory function via adenosinergic mechanisms at 2 weeks post-injury (the time-point serotonergic pathways are still impaired) and via serotonergic mechanisms at 8 weeks post-injury (the time-point serotonergic pathways are partially recovered). Thus, serotonin plays a significant role in plasticity following cervical hemisection injuries, however, these findings should be tested in cervical spinal contusion.
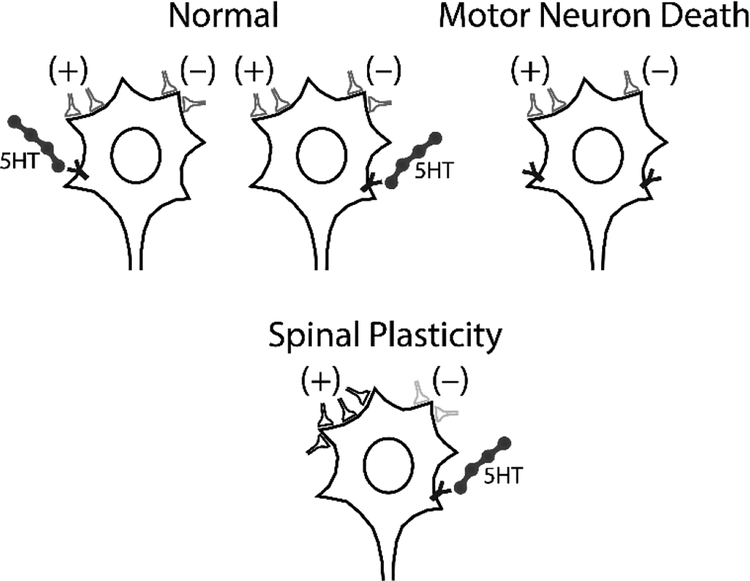
Involvement of interneurons was implicated in spinal compensatory processes after cervical contusion injury using pseudo-rabies virus (59) and C2 hemisection injury using Alexa-488 conjugated wheat germ agglutinin (96). Both studies reported higher number of interneurons innervating the phrenic motor neurons, consistent with increased terminal sprouting or synaptic strengthening. A recent study support functional significance of excitatory interneurons in recovery of function after cervical SCI. While VGLUT2+ excitatory interneurons are not necessary for normal breathing, they increase their innervation of phrenic motor neurons and contribute to breathing after non-traumatic spinal cord compression injury (97). A similar effect is observed with inhibition of inhibitory neurotransmission in the spinal cord. For example, a latent spinal network generating phrenic bursting is revealed after inhibition of GABAergic and glycinergic transmission suggesting that inhibitory interneuronal networks can be harnessed to promote spinal compensatory plasticity (98). Furthermore, interneurons may also underlie/mediate the contralateral compensatory muscle activity following lateralized injuries such as spinal hemisection (40, 44) and unilateral denervation (99). Thus, it is likely that interneurons play a significant functional role in respiratory motor network via a suite of mechanisms enhancing respiratory motor function (Figure 2, see review (100).
Figure 2.
Spinal Plasticity after Motor Neuron Death. To preserve ventilatory and airway protective functions of respiratory motor network, the following remodeling strategies can be employed: 1) strengthening/weakening existing excitatory/inhibitory synapses and 2) form new synapses or prune existing synapses. 3) Restoration of neuromodulatory (e.g. 5HT) innervation. Consequently, phrenic nerve output can be at least partially be restored following phrenic motor neuron death.
3.3. IMPROVED NEUROMUSCULAR TRANSMISSION
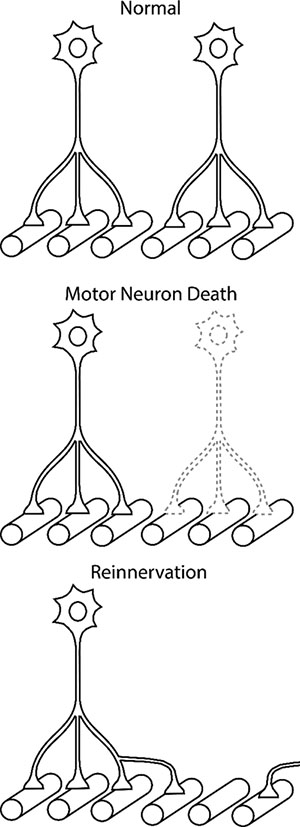
There is a one-to-one relationship between motor neuron discharge and muscle fiber activity. Each time a motor neuron discharges, the muscle fibers it innervates are also activated. However, with progressive motor neuron death, denervated muscle fibers remain silent until they are reinnervated by surviving motor neurons via end-terminal sprouting (Figure 3). A motor neuron can increase the number of muscle fibers it innervates (i.e. innervation ratio) by up to ~5- to 8-fold within a year (101), amplifying neural output to the muscle and effectively compensating for early-stages of motor neuron loss both in animal models and clinical populations (102). This compensatory mechanism has may have the advantage that the same glutamatergic input to motor neurons can generate greater force without inducing excitotoxicity.
Figure 3.
Increased Motor Neuron Terminal Sprouting after Motor Neuron Death. In healthy adults, each muscle fiber is innervated by a single motor neuron. With neurodegenerative disease or trauma, some motor neurons die (or undergo axonal detachment) and their muscle fibers are denervated. The adjacent motor neuron terminals sprout to reinnervate the denervated muscle fibers. As the pathology progresses, the number of muscle fibers innervated by each motor neuron increases up to 5- to 8-fold preserving ventilatory and airway protective functions of respiratory motor network, However, further sprouting and increased discharge activity may lead to neuromuscular transmission problems and muscle dysfunction.
In SOD1G93A rats, although only ~50% of phrenic nerve activity is maintained, diaphragm EMG activity is fully preserved (21, 22) consistent with improved neuromuscular transmission from phrenic nerve to diaphragm muscle. Increased motor unit innervation ratio (i.e. average number of muscle fibers innervated per motor neuron) is mediated by axon terminal sprouting and motor end-plate reinnervation (103). In pre-symptomatic SOD1G93A mice, the tibialis anterior innervation ratio was increased by 44% (26). We speculate that diaphragm muscle innervation ratio may further increase until the end-stage ALS, compensating for phrenic motor neuron death. Unfortunately, as each surviving motor neuron expands its muscle fiber innervation, it has more neuromuscular junctions to maintain via axonal transport, which can be severely impaired in neurodegenerative diseases and/or neurotrauma (104). Extensive sprouting observed when motor neuron loss or muscle fiber denervation exceeds 85% and increased duty cycle for motor neuron discharge increase oxidative stress, which impairs sprouting capacity and may weaken existing and newly innervated presynaptic terminals leading to neuromuscular transmission fatigue and decreased muscle force generation (102, 105, 106). Regardless of preserved diaphragm EMG activity, repeated denervation and reinnervation cycles likely cause muscle fiber atrophy. In pre-symptomatic SOD1G93A mice, force per muscle fiber decreased ~60% (26). Consequently, trans-diaphragmatic pressure (in vivo surrogate of diaphragm force) in rats was reduced by ~30%, suggesting secondary diaphragm dysfunction (21).
The same compensatory strategy was observed in the rat model of cervical contusion injury. Following cervical contusion, an initial reduction in diaphragm compound muscle action potential (CMAP) amplitudes was partially recovered over time consistent with partial reinnervation of diaphragm motor end plates (54). However, diaphragm CMAP was not fully restored in agreement with the observation of immature neuromuscular junctions as evident by high percentage of partially or multiply innervated diaphragm motor end plates.
3.4. ACCESSORY RESPIRATORY MUSCLE RECRUITMENT
Diaphragm muscle dysfunction or paralysis elicits compensatory increases in the activity of accessory respiratory muscles (e.g. intercostal, neck, abdominal muscles), in part due to the release of inhibition originating from phrenic afferents (107). Neck muscle recruitment following diaphragm paralysis is vital in people with ALS. Preserved inspiratory sternocleidomastoid activation during REM sleep is associated with longer REM sleep duration in ALS patients with diaphragmatic dysfunction (108). On the other hand, sternocleidomastoid muscle weakness is associated with lower sniff and maximum inspiratory pressures in ALS patients (109). Neck muscle weakness is the most significant prognostic factor for the necessity for mechanical ventilation or death in ALS patients (110). Other neuromuscular disorders such as Pompe disease and Duchenne muscular dystrophy require accessory muscle function to avoid ventilatory insufficiency (111, 112).
Accessory muscle recruitment is a compensatory response utilized during other pathological conditions involving diaphragm paralysis (107, 113–116). In SOD1G93A rats, trans-diaphragmatic pressure is impaired at the late stages of disease leading to powerful recruitment of external intercostal muscles that are normally quiescent, likely compensating for diaphragm dysfunction (21). A class of glutamatergic neurons (V2a neurons) were implicated in the recruitment of accessory respiratory muscles in SOD1G93A mice suggesting that degeneration of V2a neurons would induce decompensation and likely lead to respiratory failure (117).
Compensatory intercostal muscle activation is also utilized in rodent models of cervical contusion injury as early as 20 min post-injury (42). However, increased intercostal muscle activation was far from reaching its functional reserve, which is utilized via therapeutic modalities. In fact, intercostal muscle pacing via epidural stimulation was reported to be beneficial in ventilator-dependent patients (118). When combined with diaphragm pacing, intercostal muscle pacing enabled ventilator-dependent patients to forego mechanical ventilation for 16 to 24 hours per day (119).
4. CONCLUSION
Respiratory motor neuron loss imposes major challenges to the respiratory control system. Powerful and diverse compensatory responses preserve ventilatory capacity, even with major motor neuron death. However, as metabolic workload of spared motor neurons increase, maladaptive decompensation may accelerate motor neuron death, leading patients to sudden respiratory failure. Here, we outline spontaneous compensatory processes preserving ventilatory capacity, despite their potential to compromise motor neuron survival. This appears to be a decision between preservation of life for the patient or animal model versus accelerated motor neuron death. Improved understanding of these processes may enable development of new strategies to slow progression towards respiratory impairment with neurological injury or disease.
HIGHLIGHTS.
Multiple neuromuscular disorders lead to loss of respiratory motor neurons.
Mechanisms of compensatory plasticity prevent respiratory failure in the presymptomatic stages of pathology.
As the pathology aggravates, compensatory plasticity reaches its limits.
Increased metabolic demand and discharge activity accelerate the demise of surviving motor neurons.
Acknowledgments
Sources of Funding: Support provided by NIH HL69064 and the McKnight Brain Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no competing financial interests.
References
- 1.Kennedy PG. On the possible role of viruses in the aetiology of motor neurone disease: a review. J R Soc Med 1990; 83: 784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson RA, Mitchell GS. Common mechanisms of compensatory respiratory plasticity in spinal neurological disorders. Respir Physiol Neurobiol 2013; 189: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 2010; 173: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet 2011; 377: 942–955. [DOI] [PubMed] [Google Scholar]
- 5.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011; 72: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karamyan VT, Speth RC. Animal models of BMAA neurotoxicity: a critical review. Life Sci 2008; 82: 233–246. [DOI] [PubMed] [Google Scholar]
- 7.Lyall RA, Donaldson N, Polkey MI, Leigh PN, Moxham J. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain 2001; 124: 2000–2013. [DOI] [PubMed] [Google Scholar]
- 8.Polkey MI, Lyall RA, Green M, Nigel Leigh P, Moxham J. Expiratory muscle function in amyotrophic lateral sclerosis. Am J Respir Crit Care Med 1998; 158: 734–741. [DOI] [PubMed] [Google Scholar]
- 9.Bourke SC, Shaw PJ, Gibson GJ. Respiratory function vs sleep-disordered breathing as predictors of QOL in ALS. Neurology 2001; 57: 2040–2044. [DOI] [PubMed] [Google Scholar]
- 10.Fallat RJ, Jewitt B, Bass M, Kamm B, Norris FH. Spirometry in amyotrophic lateral sclerosis. Arch Neurol 1979; 36: 74–80. [DOI] [PubMed] [Google Scholar]
- 11.Park SY, Kim SM, Sung JJ, Lee KM, Park KS, Kim SY, Nam HW, Lee KW. Nocturnal hypoxia in ALS is related to cognitive dysfunction and can occur as clusters of desaturations. PLoS One 2013; 8: e75324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson KA, Strong MJ, Ahmad D, George CF. Sleep-disordered breathing in amyotrophic lateral sclerosis. Chest 1996; 110: 664–669. [DOI] [PubMed] [Google Scholar]
- 13.Chetta A, Aiello M, Tzani P, Olivieri D. Assessment and monitoring of ventilatory function and cough efficacy in patients with amyotrophic lateral sclerosis. Monaldi Arch Chest Dis 2007; 67: 43–52. [DOI] [PubMed] [Google Scholar]
- 14.Plowman EK, Watts SA, Robison R, Tabor L, Dion C, Gaziano J, Vu T, Gooch C. Voluntary Cough Airflow Differentiates Safe Versus Unsafe Swallowing in Amyotrophic Lateral Sclerosis. Dysphagia 2016; 31: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993; 362: 59–62. [DOI] [PubMed] [Google Scholar]
- 16.Lladó J, Haenggeli C, Pardo A, Wong V, Benson L, Coccia C, Rothstein JD, Shefner JM, Maragakis NJ. Degeneration of respiratory motor neurons in the SOD1 G93A transgenic rat model of ALS. Neurobiol Dis 2006; 21: 110–118. [DOI] [PubMed] [Google Scholar]
- 17.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci U S A 2002; 99: 1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 1995; 14: 1105–1116. [DOI] [PubMed] [Google Scholar]
- 19.Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH, Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci 2001; 21: 9246–9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN, Mitchell GS. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am J Respir Crit Care Med 2013; 187: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seven YB, Nichols NL, Kelly MN, Hobson OR, Satriotomo I, Mitchell GS. Compensatory plasticity in diaphragm and intercostal muscle utilization in a rat model of ALS. Exp Neurol 2018; 299: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols NL, Van Dyke J, Nashold L, Satriotomo I, Suzuki M, Mitchell GS. Ventilatory control in ALS. Respir Physiol Neurobiol 2013; 189: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tankersley CG, Haenggeli C, Rothstein JD. Respiratory impairment in a mouse model of amyotrophic lateral sclerosis. J Appl Physiol (1985) 2007; 102: 926–932. [DOI] [PubMed] [Google Scholar]
- 24.Seven YB, Mantilla CB, Sieck GC. Recruitment of rat diaphragm motor units across motor behaviors with different levels of diaphragm activation. J Appl Physiol (1985) 2014; 117: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol 2013; 185: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol 2008; 586: 3337–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 2007; 28: 154–164. [DOI] [PubMed] [Google Scholar]
- 28.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 2004; 185: 232–240. [DOI] [PubMed] [Google Scholar]
- 29.Nichols NL, Vinit S, Bauernschmidt L, Mitchell GS. Respiratory function after selective respiratory motor neuron death from intrapleural CTB-saporin injections. Exp Neurol 2014; 267: 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 2009; 182: 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolognesi A, Tazzari PL, Olivieri F, Polito L, Falini B, Stirpe F. Induction of apoptosis by ribosomeinactivating proteins and related immunotoxins. Int J Cancer 1996; 68: 349–355. [DOI] [PubMed] [Google Scholar]
- 32.Llewellyn-Smith IJ, Martin CL, Arnolda LF, Minson JB. Retrogradely transported CTB-saporin kills sympathetic preganglionic neurons. Neuroreport 1999; 10: 307–312. [DOI] [PubMed] [Google Scholar]
- 33.Llewellyn-Smith IJ, Martin CL, Arnolda LF, Minson JB. Tracer-toxins: cholera toxin B-saporin as a model. J Neurosci Methods 2000; 103: 83–90. [DOI] [PubMed] [Google Scholar]
- 34.Frankel HL, Coll JR, Charlifue SW, Whiteneck GG, Gardner BP, Jamous MA, Krishnan KR, Nuseibeh I, Savic G, Sett P. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord 1998; 36: 266–274. [DOI] [PubMed] [Google Scholar]
- 35.DeVivo MJ, Ivie CS. Life expectancy of ventilator-dependent persons with spinal cord injuries. Chest 1995; 108: 226–232. [DOI] [PubMed] [Google Scholar]
- 36.Mansel JK, Norman JR. Respiratory complications and management of spinal cord injuries. Chest 1990; 97: 1446–1452. [DOI] [PubMed] [Google Scholar]
- 37.Khurram OU, Fogarty MJ, Rana S, Vang P, Sieck GC, Mantilla CB. Diaphragm muscle function following mid-cervical contusion injury in rats. J Appl Physiol (1985) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol 2009; 169: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol (1985) 2003; 94: 795–810. [DOI] [PubMed] [Google Scholar]
- 40.Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol (1985) 2013; 114: 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol 2009; 169: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen MH, Lee KZ. Diaphragm and Intercostal Muscle Activity after Mid-Cervical Spinal Cord Contusion in the Rat. J Neurotrauma 2018; 35: 533–547. [DOI] [PubMed] [Google Scholar]
- 43.Lee KZ. Impact of cervical spinal cord contusion on the breathing pattern across the sleep-wake cycle in the rat. J Appl Physiol (1985) 2018. [DOI] [PubMed] [Google Scholar]
- 44.Lee KZ, Hsu SH. Compensatory Function of the Diaphragm after High Cervical Hemisection in the Rat. J Neurotrauma 2017; 34: 2634–2644. [DOI] [PubMed] [Google Scholar]
- 45.Lee KZ, Kuo HC. Vagal Control of Breathing Pattern after Midcervical Contusion in Rats. J Neurotrauma 2017; 34: 734–745. [DOI] [PubMed] [Google Scholar]
- 46.Kang SW, Shin JC, Park CI, Moon JH, Rha DW, Cho DH. Relationship between inspiratory muscle strength and cough capacity in cervical spinal cord injured patients. Spinal Cord 2006; 44: 242–248. [DOI] [PubMed] [Google Scholar]
- 47.Bascom AT, Sankari A, Goshgarian HG, Badr MS. Sleep onset hypoventilation in chronic spinal cord injury. Physiol Rep 2015; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berlowitz DJ, Brown DJ, Campbell DA, Pierce RJ. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil 2005; 86: 1193–1199. [DOI] [PubMed] [Google Scholar]
- 49.Sajkov D, Marshall R, Walker P, Mykytyn I, McEvoy RD, Wale J, Flavell H, Thornton AT, Antic R. Sleep apnoea related hypoxia is associated with cognitive disturbances in patients with tetraplegia. Spinal Cord 1998; 36: 231–239. [DOI] [PubMed] [Google Scholar]
- 50.Grumbles RM, Thomas CK. Motoneuron Death after Human Spinal Cord Injury. J Neurotrauma 2017; 34: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castro MJ, Apple DF, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol (1985) 1999; 86: 350–358. [DOI] [PubMed] [Google Scholar]
- 52.Thomas CK, Zaidner EY, Calancie B, Broton JG, Bigland-Ritchie BR. Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp Neurol 1997; 148: 414–423. [DOI] [PubMed] [Google Scholar]
- 53.Nicaise C, Hala TJ, Frank DM, Parker JL, Authelet M, Leroy K, Brion JP, Wright MC, Lepore AC. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp Neurol 2012; 235: 539–552. [DOI] [PubMed] [Google Scholar]
- 54.Nicaise C, Frank DM, Hala TJ, Authelet M, Pochet R, Adriaens D, Brion JP, Wright MC, Lepore AC. Early phrenic motor neuron loss and transient respiratory abnormalities after unilateral cervical spinal cord contusion. J Neurotrauma 2013; 30: 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rana S, Sieck GC, Mantilla CB. Diaphragm electromyographic activity following unilateral midcervical contusion injury in rats. J Neurophysiol 2017; 117: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicaise C, Putatunda R, Hala TJ, Regan KA, Frank DM, Brion JP, Leroy K, Pochet R, Wright MC, Lepore AC. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following midcervical spinal contusion in mice. J Neurotrauma 2012; 29: 2748–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Golder FJ, Fuller DD, Lovett-Barr MR, Vinit S, Resnick DK, Mitchell GS. Breathing patterns after midcervical spinal contusion in rats. Exp Neurol 2011; 231: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warren PM, Campanaro C, Jacono FJ, Alilain WJ. Mid-cervical spinal cord contusion causes robust deficits in respiratory parameters and pattern variability. Exp Neurol 2018; 306: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lane MA, Lee KZ, Salazar K, O’Steen BE, Bloom DC, Fuller DD, Reier PJ. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp Neurol 2012; 235: 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan S, Plummer M, Martinez-Arizala A, Banovac K. Hypothermia in patients with chronic spinal cord injury. J Spinal Cord Med 2007; 30: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt KD, Chan CW. Thermoregulation and fever in normal persons and in those with spinal cord injuries. Mayo Clin Proc 1992; 67: 469–475. [DOI] [PubMed] [Google Scholar]
- 62.DRORBAUGH JE FENN WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 1955; 16: 81–87. [PubMed] [Google Scholar]
- 63.Lewerenz J, Maher P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front Neurosci 2015; 9: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal 2009; 11: 1587–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaw PJ, Ince PG. Glutamate, excitotoxicity and amyotrophic lateral sclerosis. J Neurol 1997; 244 Suppl 2: S3–14. [DOI] [PubMed] [Google Scholar]
- 66.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 1996; 16: 675–686. [DOI] [PubMed] [Google Scholar]
- 67.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 1997; 18: 327–338. [DOI] [PubMed] [Google Scholar]
- 68.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med 1992; 326: 1464–1468. [DOI] [PubMed] [Google Scholar]
- 69.Martin LJ, Chang Q. Inhibitory synaptic regulation of motoneurons: a new target of disease mechanisms in amyotrophic lateral sclerosis. Mol Neurobiol 2012; 45: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang Q, Martin LJ. Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a quantitative confocal analysis. Am J Pathol 2009; 174: 574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, Golden WC. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol 2007; 500: 20–46. [DOI] [PubMed] [Google Scholar]
- 72.Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther 2011; 17: 4–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doble A The pharmacology and mechanism of action of riluzole. Neurology 1996; 47: S233–241. [DOI] [PubMed] [Google Scholar]
- 74.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci 2010; 1198: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 2010; 669: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turner S, Streeter KA, Greer J, Mitchell GS, Fuller DD. Pharmacological modulation of hypoxia-induced respiratory neuroplasticity. Respir Physiol Neurobiol 2018; 256: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 2008; 28: 2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 2009; 587: 5469–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol 2010; 588: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 2011; 589: 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dale EA, Satriotomo I, Mitchell GS. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J Neurosci 2012; 32: 5973–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 2011; 31: 7682–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol (1985) 2012; 112: 1678–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huxtable AG, MacFarlane PM, Vinit S, Nichols NL, Dale EA, Mitchell GS. Adrenergic α₁ receptor activation is sufficient, but not necessary for phrenic long-term facilitation. J Appl Physiol (1985) 2014; 116: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seven YB, Perim RR, Hobson OR, Simon AK, Tadjalli A, Mitchell GS. Phrenic motor neuron adenosine 2A receptors elicit phrenic motor facilitation. J Physiol 2018; 596: 1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol (1985) 2012; 113: 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El Oussini H, Scekic-Zahirovic J, Vercruysse P, Marques C, Dirrig-Grosch S, Dieterlé S, Picchiarelli G, Sinniger J, Rouaux C, Dupuis L. Degeneration of serotonin neurons triggers spasticity in amyotrophic lateral sclerosis. Ann Neurol 2017; 82: 444–456. [DOI] [PubMed] [Google Scholar]
- 88.Dentel C, Palamiuc L, Henriques A, Lannes B, Spreux-Varoquaux O, Gutknecht L, René F, Echaniz-Laguna A, Gonzalez de Aguilar JL, Lesch KP, Meininger V, Loeffler JP, Dupuis L. Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: a link to spasticity. Brain 2013; 136: 483–493. [DOI] [PubMed] [Google Scholar]
- 89.Nichols NL, Satriotomo I, Allen LL, Grebe AM, Mitchell GS. Mechanisms of Enhanced Phrenic Long-Term Facilitation in SOD1(G93A) Rats. J Neurosci 2017; 37: 5834–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nichols NL, Craig TA, Tanner MA. Phrenic long-term facilitation following intrapleural CTB-SAP-induced respiratory motor neuron death. Respir Physiol Neurobiol 2018; 256: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fields DP, Mitchell GS. Spinal metaplasticity in respiratory motor control. Front Neural Circuits 2015; 9: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 2005; 25: 2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol (1985) 2001; 91: 2665–2673. [DOI] [PubMed] [Google Scholar]
- 94.Teng YD, Bingaman M, Taveira-DaSilva AM, Pace PP, Gillis RA, Wrathall JR. Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci 2003; 23: 4182–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med 2006; 29: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buttry JL, Goshgarian HG. Injection of WGA-Alexa 488 into the ipsilateral hemidiaphragm of acutely and chronically C2 hemisected rats reveals activity-dependent synaptic plasticity in the respiratory motor pathways. Exp Neurol 2014; 261: 440–450. [DOI] [PubMed] [Google Scholar]
- 97.Satkunendrarajah K, Karadimas SK, Laliberte AM, Montandon G, Fehlings MG. Cervical excitatory neurons sustain breathing after spinal cord injury. Nature 2018; 562: 419–422. [DOI] [PubMed] [Google Scholar]
- 98.Cregg JM, Chu KA, Hager LE, Maggard RSJ, Stoltz DR, Edmond M, Alilain WJ, Philippidou P, Landmesser LT, Silver J. A Latent Propriospinal Network Can Restore Diaphragm Function after High Cervical Spinal Cord Injury. Cell Rep 2017; 21: 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khurram OU, Sieck GC, Mantilla CB. Compensatory effects following unilateral diaphragm paralysis. Respir Physiol Neurobiol 2017; 246: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zholudeva LV, Qiang L, Marchenko V, Dougherty KJ, Sakiyama-Elbert SE, Lane MA. The Neuroplastic and Therapeutic Potential of Spinal Interneurons in the Injured Spinal Cord. Trends Neurosci 2018; 41: 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gordon T, Tyreman N. Sprouting capacity of lumbar motoneurons in normal and hemisected spinal cords of the rat. J Physiol 2010; 588: 2745–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gordon T, Hegedus J, Tam SL. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res 2004; 26: 174–185. [DOI] [PubMed] [Google Scholar]
- 103.Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol 2005; 490: 209–219. [DOI] [PubMed] [Google Scholar]
- 104.De Vos KJ, Hafezparast M. Neurobiology of axonal transport defects in motor neuron diseases: Opportunities for translational research? Neurobiol Dis 2017; 105: 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rizzuto E, Pisu S, Musarò A, Del Prete Z. Measuring Neuromuscular Junction Functionality in the SOD1(G93A) Animal Model of Amyotrophic Lateral Sclerosis. Ann Biomed Eng 2015; 43: 2196–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rafuse VF, Gordon T, Orozco R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. J Neurophysiol 1992; 68: 1261–1276. [DOI] [PubMed] [Google Scholar]
- 107.Brichant JF, De Troyer A. On the intercostal muscle compensation for diaphragmatic paralysis in the dog. J Physiol 1997; 500 (Pt 1): 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arnulf I, Similowski T, Salachas F, Garma L, Mehiri S, Attali V, Behin-Bellhesen V, Meininger V, Derenne JP. Sleep disorders and diaphragmatic function in patients with amyotrophic lateral sclerosis. Am J Respir Crit Care Med 2000; 161: 849–856. [DOI] [PubMed] [Google Scholar]
- 109.Pinto S, de Carvalho M. Motor responses of the sternocleidomastoid muscle in patients with amyotrophic lateral sclerosis. Muscle Nerve 2008; 38: 1312–1317. [DOI] [PubMed] [Google Scholar]
- 110.Nakamura R, Atsuta N, Watanabe H, Hirakawa A, Ito M, Senda J, Katsuno M, Tanaka F, Izumi Y, Morita M, Ogaki K, Taniguchi A, Aiba I, Mizoguchi K, Okamoto K, Hasegawa K, Aoki M, Kawata A, Abe K, Oda M, Konagaya M, Imai T, Nakagawa M, Tsuji S, Kaji R, Nakano I, Sobue G. Neck weakness is a potent prognostic factor in sporadic amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry 2013; 84: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 111.Carlier RY, Laforet P, Wary C, Mompoint D, Laloui K, Pellegrini N, Annane D, Carlier PG, Orlikowski D. Whole-body muscle MRI in 20 patients suffering from late onset Pompe disease: Involvement patterns. Neuromuscul Disord 2011; 21: 791–799. [DOI] [PubMed] [Google Scholar]
- 112.Barbé F, Quera-Salva MA, McCann C, Gajdos P, Raphael JC, de Lattre J, Agustí AG. Sleep-related respiratory disturbances in patients with Duchenne muscular dystrophy. Eur Respir J 1994; 7: 1403–1408. [DOI] [PubMed] [Google Scholar]
- 113.Katagiri M, Young RN, Platt RS, Kieser TM, Easton PA. Respiratory muscle compensation for unilateral or bilateral hemidiaphragm paralysis in awake canines. J Appl Physiol (1985) 1994; 77: 1972–1982. [DOI] [PubMed] [Google Scholar]
- 114.Maskrey M, Megirian D, Sherrey JH. Alteration in breathing of the awake rat after laryngeal and diaphragmatic muscle paralysis. Respir Physiol 1990; 81: 203–212. [DOI] [PubMed] [Google Scholar]
- 115.Ninane V, Farkas GA, Baer R, de Troyer A. Mechanism of rib cage inspiratory muscle recruitment in diaphragmatic paralysis. Am Rev Respir Dis 1989; 139: 146–149. [DOI] [PubMed] [Google Scholar]
- 116.Teitelbaum J, Borel CO, Magder S, Traystman RJ, Hussain SN. Effect of selective diaphragmatic paralysis on the inspiratory motor drive. J Appl Physiol (1985) 1993; 74: 2261–2268. [DOI] [PubMed] [Google Scholar]
- 117.Romer SH, Seedle K, Turner SM, Li J, Baccei ML, Crone SA. Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Exp Neurol 2017; 287: 192–204. [DOI] [PubMed] [Google Scholar]
- 118.DiMarco AF, Supinski GS, Petro JA, Takaoka Y. Evaluation of intercostal pacing to provide artificial ventilation in quadriplegics. Am J Respir Crit Care Med 1994; 150: 934–940. [DOI] [PubMed] [Google Scholar]
- 119.DiMarco AF, Takaoka Y, Kowalski KE. Combined intercostal and diaphragm pacing to provide artificial ventilation in patients with tetraplegia. Arch Phys Med Rehabil 2005; 86: 1200–1207. [DOI] [PubMed] [Google Scholar]