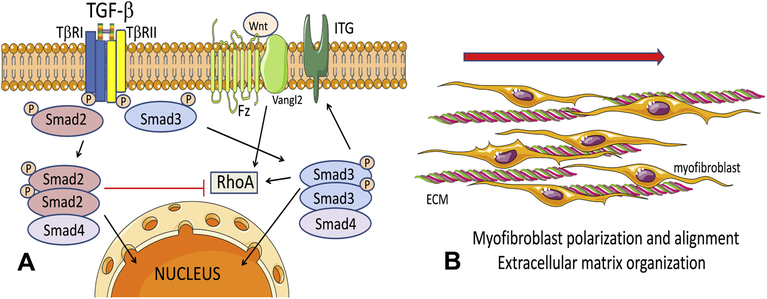
Figure 8: Schematic cartoons illustrate the contrasting roles of Smad2 and Smad3 in activation of infarct myofibroblasts following myocardial infarction.
A: TGF-βs bind and sequentially transphosphorylate type I and type II TGF-β receptors (TβRI, TβRII respectively), leading to downstream phosphorylation of Smad2 and Smad3. Activated Smad2 and Smad3 form complexes with the common Smad, Smad4 and translocate to the nucleus, where they may interact with a wide range of transcriptional activators and repressors and regulate transcription. In activated fibroblasts, Smad3 mediates expression of surface integrins (ITG), promoting interactions with other cells and the extracellular matrix (ECM). Moreover, Smad3 mediates expression of RhoA, a downstream effector of the Wnt/Fz/Vangl2 PCP pathway that may promote fibroblast alignment. In contrast, Smad2 does not critically affect α2 and α5 integrin expression and downmodulates RhoA synthesis. The distinct effects of Smad2 and Smad3 may explain the in vivo phenotypes observed in mice with fibroblast-specific Smad2 and Smad3 loss. Smad3 knockdown, but not Smad2 loss, perturbs myofibroblast polarization and formation of aligned myofibroblast arrays in the infarct, a process critical for organization of the healing scar (B).