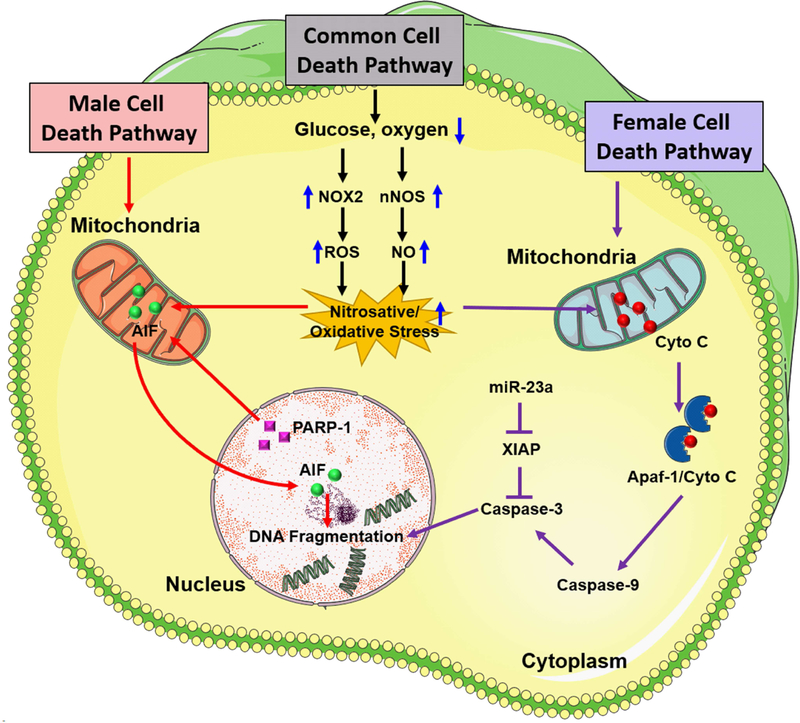
Figure 1:
Sex differences in cell death pathways that contribute to ischemic brain damage. Following focal ischemia, caspase-independent cell death pathway mediated by PARP-1 activation and nuclear translocation of AIF is predominant in males whereas caspase activation predominantly mediates ischemic neuronal death in the female brain. After an ischemic insult, nNOS and NOX2 activation promote high levels of nitric oxide (NO) formation which interacts with superoxide to produce highly reactive peroxynitrite free radicals. Once formed, peroxynitrite free radicals damage protein, lipids as well as DNA, eventually resulting in activation of PARP-1, a DNA repair enzyme that protects the genome under normal physiologic conditions. PARP-1, in turn, induces the release of AIF from mitochondria and its translocation to the nucleus. Overactivation of PARP-1 and nuclear translocation of AIF together promote DNA fragmentation and initiation of caspase-independent cell death pathway in males. In females, ischemia-induced oxidative stress leads to release of mitochondrial cytochrome C into the cytosol which is a hallmark of caspase activation via induction of mitochondrial permeability transition pore (mPTP). In the cytosol, cytochrome C interacts with apoptotic protease activating factor-1 (Apaf-1) to form an Apaf-1/Cyto-C complex in an ATP-dependent manner. This apoptosome complex cleaves procaspase-9 to active caspase-9 which induces activation of effector caspases including caspase-3 which mediates cleavage of most apoptotic substrates including the inhibitor of caspase-activated DNase leading to DNA fragmentation. X-linked inhibitor of apoptosis (XIAP) is a known inhibitor of caspases in females that is regulated by miR-23a.