Figure 1.
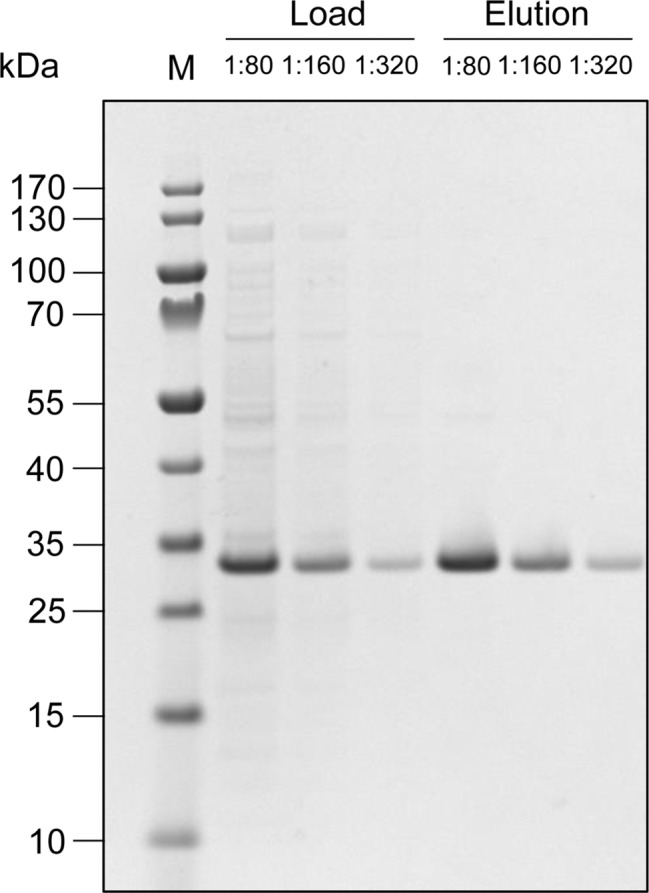
Production of a 19-kDa phenylalanine-free protein in P. fluorescens. After extraction and the removal of cell debris, the product was purified from the clarified extract by single-stage immobilized metal-ion affinity chromatography. Due to the high protein concentration, representative samples of the load and elution fraction were analyzed by SDS-PAGE at dilution ratios of 1:80, 1:160, and 1:320. The strong band at ~32 kDa represents the phenylalanine-free protein – the larger size of the protein probably reflects a combination of its high surface charge and generally high stability, which prevents full de-folding during sample preparation. This example shows the high efficiency of the purification step: only traces of other proteins are found in addition to the target protein in the final elution fraction, corresponding to a purity of >95%.
