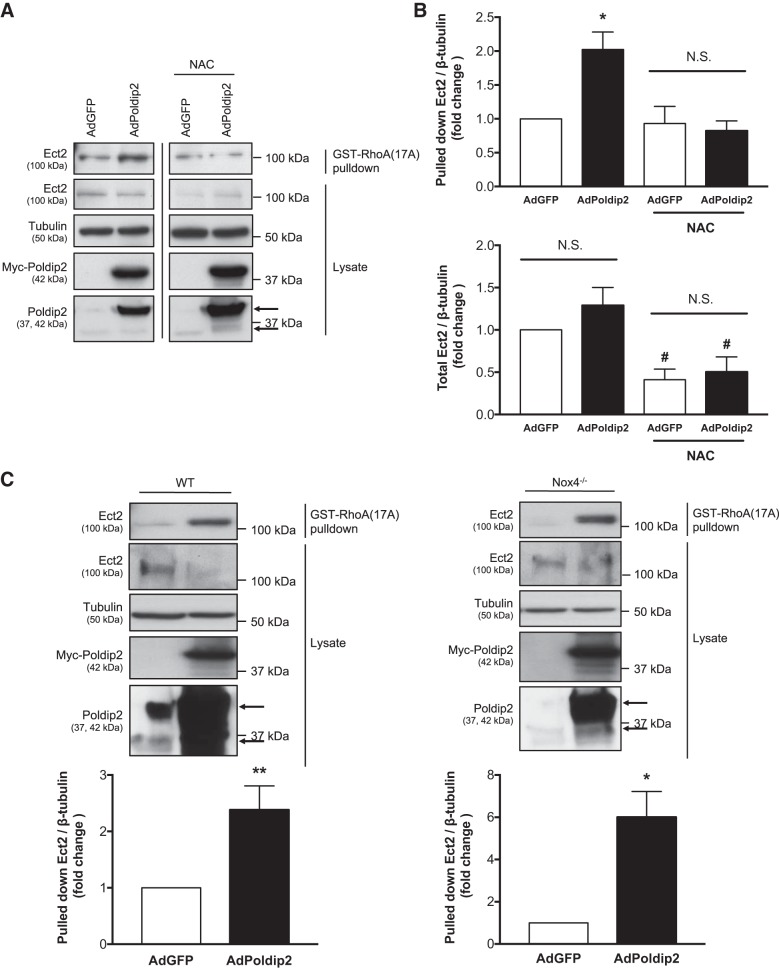
Fig. 3.
Reactive oxygen species, but not Nox4, are required for polymerase-δ-interacting protein 2 (Poldip2)-mediated epithelial cell transforming sequence 2 (Ect2) activity. A: rat aortic smooth muscle cells (RASMs) were transduced with Myc-Poldip2 (AdPoldip2) or vector-only (AdGFP) adenovirus and the following day treated with 10–20 mM N-acetyl-cysteine (NAC). Twenty-four hours later, GST-RhoA(17A) pulldowns for active guanine nucleotide exchange factors (GEFs) were performed and immunoblotted for Ect2. Prior to pulldown, some lysate was retained to examine total Ect2 and confirm even loading and overexpression. The top arrow identifies exogenous Poldip2, and bottom arrow identifies endogenous Poldip2 on the Poldip2 immunoblot. Representative immunoblots are from the same film and exposure for each blotted protein. The vertical black line between lanes indicates cropping of extraneous lanes. B: the average amount of pulled down and total Ect2 normalized to tubulin is shown (n = 3–6; *P < 0.05 vs. AdGFP; #P < 0.05 vs. no NAC ratio paired t-test; N.S., not significant; error bars represent SE). C: Similarly, wild-type (WT) and Nox4 knockout (Nox4−/−) mouse aortic smooth muscle cells (MASMs) were transduced with Myc-Poldip2 (AdPoldip2) or vector-only (AdGFP) adenovirus and GST-RhoA(17A) pulldowns for active GEFs were performed and immunoblotted for Ect2. The average amount of pulled down Ect2 normalized to tubulin is shown as a fold change above AdGFP (n = 3–7; *P < 0.05; **P < 0.01 ratio paired t-test; error bars represent SE).