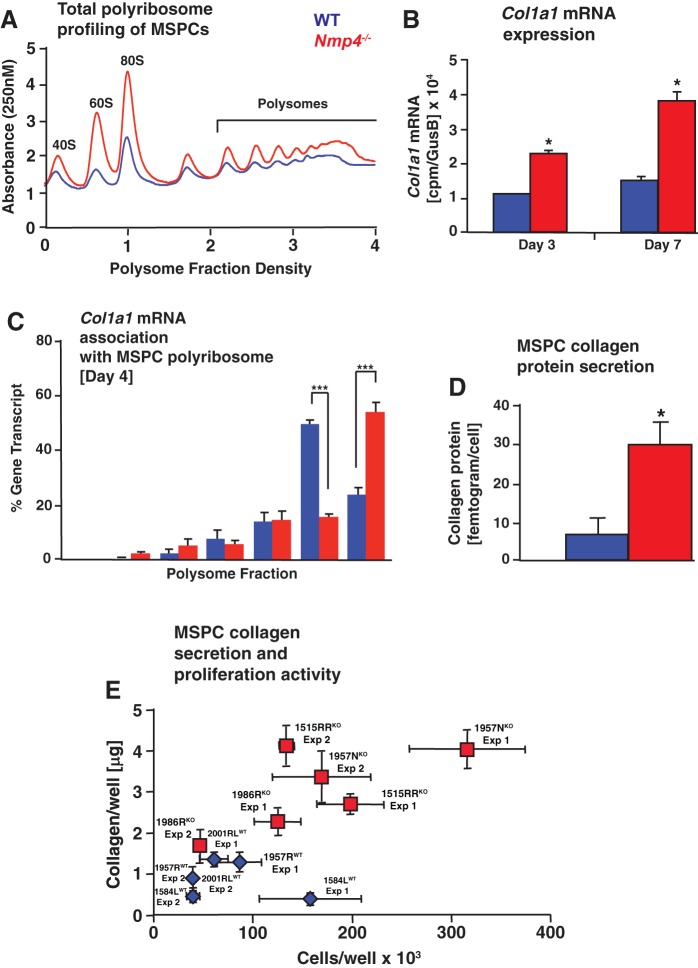
Fig. 10.
Loss of nuclear matrix protein 4 (Nmp4) enhances collagen expression and secretion. Data were derived from mesenchymal stem/progenitor cell (MSPC) preparations 1584LWT, 1515RRKO, 1957RWT, and 1957NKO. A: polysome profiles of lysates prepared from wild-type (WT) and Nmp4−/− MSPCs at 4 days in culture. Representative profiles from 3 biological replicates. B: Col1a1 mRNA expression as determine by RNA sequencing (RNA-seq) in MSPCs maintained in nondifferentiation medium for 3 days in culture and 7 days in culture (5 days in osteogenic medium). C: following polysome profiling, fractions 1–7 were collected, and the percentage of Col1a1 mRNA present in each sucrose gradient fraction were quantified by quantitative RT-PCR and presented as a histogram. Data are representative of 2 biological replicates and 3 technical replicates each. Statistical analyses were performed using one-way ANOVA tests; ***P < 0.0001. D: secretion of collagen protein was measured in the acid-soluble cell-matrix layer of 1584LWT and 1515RRKO by using the Sircol assay as described in materials and methods. Loss of Nmp4 significantly enhanced the amount of collagen protein secreted/cell. * P < 0.0001. Data represent 3 biological replicates and 5–6 technical replicates each. E: secretion of collagen protein was measured in the acid-soluble cell-matrix layer by using the Sircol assay at day 4 postseeding from all MSPC preparations 1584LWT, 1957RWT, 2001RLWT, 1515RRKO, 1957NKO, and 1986RKO and presented as collagen/well (µg) versus cell number/well. All six preparations were tested independently at least twice (experiments 1 and 2) and experiments comprised 4–6 wells/preparation. All Nmp4−/− (knockout) preparations produced more collagen during the first four days of culture, regardless of cell number. Data represent average ± SD; n = 4–6 wells/group.