Fig. 1.
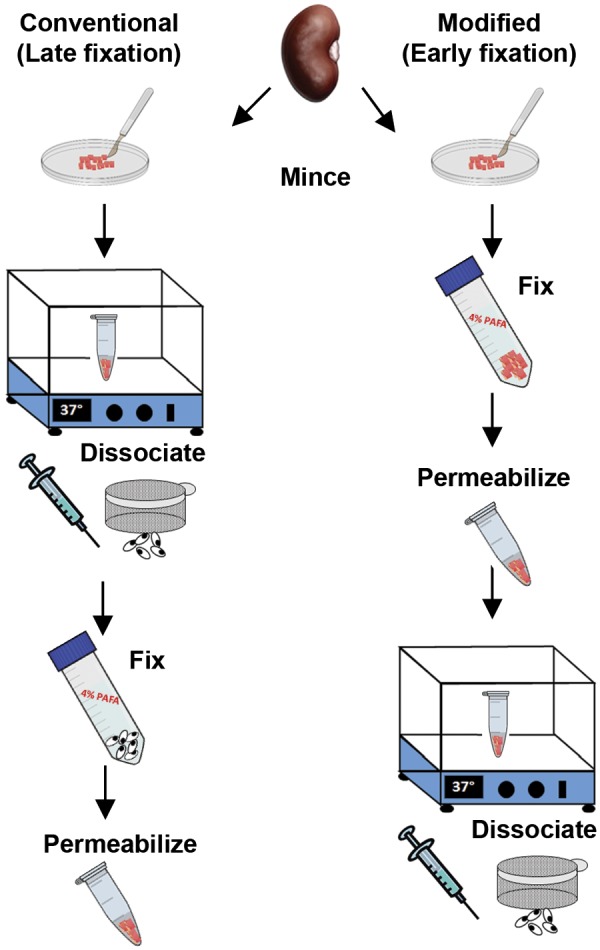
The conventional (late fixation) and modified (early fixation) protocols for kidney tissue dissociation into a single cell suspension for flow cytometry. In the conventional method, the kidney capsule is removed and tissue minced in dissociation solution (see methods) and then incubated at 37°C for 45 min. After centrifugation and shearing by a needle, the sample is filtered through a 50-μm sieve and fixed in 4% paraformaldehyde before permeabilizing in detergent buffer and washing. For the modified dissociation protocol, the tissue is minced in 4% paraformaldehyde with protease inhibitors and fixed for 30 min at room temperature, washed, incubated with the detergent solution, and then resuspended in the dissociation solution for 45 min. The tissue is passed through a 16.5-gauge needle followed by a 20-gauge needle as done in the conventional protocol and filtered through a 50-μm sieve. Please see methods for more details and listing of solutions.
