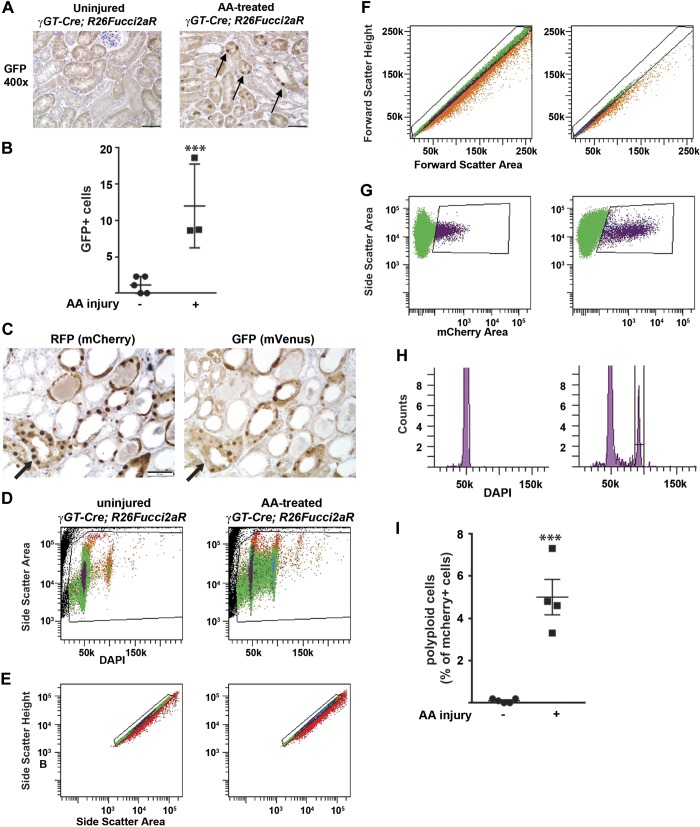
Fig. 6.
The Fucci mouse confirms increased cycling after injury and allows for analysis of polyploidy. A: uninjured and injured γ-glutamyltransferase 1 (γGT)-Cre;R26Fucci2aR mice were stained with an antibody to green fluorescent protein (GFP; cross reacts with mVenus, which indicates cells are in S/G2/M), and a representative picture is shown with a scale bar = 50 μM. Arrows point to the increased GFP+ nuclear staining in cortical tubules 7 days after aristolochic acid (AA) injury. B: GFP+ cells were counted in a blinded fashion using 10 fields (×200) per mouse (5 uninjured mice and 3 mice injured by AA 7 days prior). ***P < 0.005. C: serial sections of kidney tissue 1 wk after AA injury were stained for either red fluorescent protein (RFP; detects mCherry) or GFP (mVenus) to ensure that expression of these cell cycle reporters was mutually exclusive. Arrows point to the same tubule in serial sections with tubules that have either RFP or GFP staining but not both in the same cell. D–I: cell cycle analysis of mCherry+ (i.e., G1/G0 proximal tubules) cells from γGT-Cre;R26Fucci2aR mice was performed 1 wk after AA injury. D: the granularity channel (SSC) and nuclear stain DAPI were used to gate on nucleated cells. E and F: doublet discrimination was performed by gating on side scatter-height versus side scatter-area plot (E) as well as on forward scatter height versus forward scatter area plot (F). G: mCherry+ cells from the γGT-Cre;R26Fucci2a (γGT-Cre;Fucci) mice represent proximal tubule cells in the G1/G0 phase and can be gated on the side scatter-area versus mCherry area plot. H: representative cell cycle profiles of mCherry+ cells from uninjured and injured γGT-Cre;Fucci mice are shown. I: polyploid cells (mCherry-positive cells with 4n nuclear content) are shown in uninjured and injured mice as a percentage of mCherry+ cells.