Abstract
Circadian rhythms govern physiological functions and are important for overall health. The molecular circadian clock comprises several transcription factors that mediate circadian control of physiological function, in part, by regulating gene expression in a tissue-specific manner. These connections are well established, but the underlying mechanisms are incompletely understood. The overall goal of this study was to examine the connection among the circadian clock protein Period 1 (Per1), epithelial Na+ channel (ENaC), and blood pressure (BP) using a multipronged approach. Using global Per1 knockout mice on a 129/sv background in combination with a high-salt diet plus mineralocorticoid treatment, we demonstrated that loss of Per1 in this setting is associated with protection from hypertension. Next, we used the ENaC inhibitor benzamil to demonstrate a role for ENaC in BP regulation and urinary Na+ excretion in 129/sv mice. We targeted Per1 indirectly using pharmacological inhibition of Per1 nuclear entry in vivo to demonstrate altered expression of known Per1 target genes as well as a BP-lowering effect in 129/sv mice. Finally, we directly inhibited Per1 via genetic knockdown in amphibian distal nephron cells to demonstrate, for the first time, that reduced Per1 expression is associated with decreased ENaC activity at the single channel level.
Keywords: casein kinase 1δ/ε, circadian rhythm, epithelial Na+ channel, kidney, Period 1, strain differences
INTRODUCTION
The circadian clock mechanism evolved to allow maximum adaptation to the approximate 24-h cycle of day and night. The molecular machinery of the circadian clock is conserved across disparate kingdoms of life, from algae to bread mold to humans. Circadian rhythms are present in most physiological processes, including renal function and blood pressure (BP). Indeed, loss of circadian rhythms in cardiovascular function is associated with negative health outcomes and an increased risk for death (28, 30). Four core gene families comprise the circadian clock mechanism: brain and muscle Arnt-like protein (Bmal), Clock, Period (Per), and Cryptochrome (Cry). These genes encoding circadian clock transcription factors contribute to rhythmic changes in physiology by regulating the expression of nearly half of all genes throughout the body in a cyclical manner that repeats approximately every 24 h (32). Global knockout (KO) of any of these genes in mouse models results in alterations of BP regardless of background strain or diet and independent of changes in circadian rhythms in locomotor activity (for a review, see Ref. 5).
The renal epithelial Na+ channel (ENaC) is a key regulator of Na+ homeostasis and contributes to BP regulation by the kidney (13, 18). ENaC comprises α-, β-, and γ-subunits, and the channel is subject to multilevel regulation. ENaC is regulated at the level of expression and posttranslationally by trafficking, cleavage, and membrane recycling (for a review, see Ref. 12). The α-subunit of ENaC (αENaC) is also regulated at the level of transcription, particularly in the kidney. Per1 plays an important role in the transcriptional regulation of αENaC in collecting duct cells and in 129/sv mouse kidneys, without affecting the expression of either βENaC or γENaC (8). We have previously shown that knockout of the circadian clock gene Per1 in 129/sv mice is associated with decreased expression of αENaC in the kidney (8), increased urinary Na+ excretion (21), and lower BP in 129/sv mice (27).
The goal of the present study was to investigate the link among Per1, ENaC, and BP using a multipronged approach applied in vivo and in vitro. Based on our previous studies in 129/sv mice, we hypothesized that Per1 KO mice on this background strain would be better protected from a salt and mineralocorticoid treatment compared with wild-type (WT) mice. We further hypothesized that ENaC contributes to the hypertension observed in 129/sv mice. We directly inhibited Per1 at the genetic level using global Per1 KO mice on a 129/sv background in combination with a high-salt diet plus mineralocorticoid treatment to demonstrate that loss of Per1 in this setting is associated with protection from hypertension. Next, we used the ENaC inhibitor benzamil to demonstrate a role for ENaC in BP regulation and urinary Na+ excretion in 129/sv mice. We targeted Per1 indirectly using pharmacological inhibition of Per1 nuclear entry in vivo to demonstrate altered expression of known Per1 target genes as well as a BP-lowering effect in 129/sv mice. Finally, we directly inhibited Per1 via genetic knockdown in amphibian distal nephron cells to demonstrate, for the first time, that reduced Per1 expression is associated with decreased ENaC activity at the single channel level.
MATERIALS AND METHODS
Animals
All experiments were approved by the University of Florida and North Florida/South Georgia Veterans Affairs Medical Center Institutional Animal Care and Use Committee. All mice used in these experiments were male and were on the 129/sv background strain. WT mice were purchased from Charles River. Per1 KO mice were kindly provided by Dr. David Weaver (University of Massachusetts). Telemeter implantations surgeries were performed as previously described using PAC10 transmitters and the DSI system (27). Mice were allowed at least 7 days to recover. Four separate animal experiments were performed in independent sets of mice and are described below.
Experiment 1: high-salt diet plus mineralocorticoid treatment.
After baseline recordings on a normal diet (0.2% Na+) (these baseline data have been previously published in Ref. 27), mice were administered a high-salt diet (1% NaCl) plus mineralocorticoid. Desoxycorticosterone pivalate (DOCP) was administered via intramuscular injection. Telemetry recordings were made for 72 h (n = 6 WT mice and 5 Per1 KO mice). Data were analyzed by two-way ANOVA.
Experiment 2: benzamil treatment.
WT 129/sv mice with telemetry devices were housed in metabolic cages and spaced apart to allow for the telemetry receivers to be placed adjacent to each cage. This unique setup allowed us to simultaneously collect urine and make BP recordings, as previously described (14). Vehicle (H2O) was administered (intraperitoneally) at 8 PM, and control recordings and urine collections were made for the following 4 h. The following day at 8 PM, 1.4 mg/kg benzamil was given intraperitoneally, and recordings and urine collections were made for the following 4 h (n = 4 mice total, allowing for paired t-test comparisons between vehicle and benzamil treatment). The 1.4 mg/kg dose was based on several reports in the literature that reported an effect of benzamil on ENaC in vivo (3, 29, 33).
Experiment 3: casein kinase inhibitor treatment for gene expression experiments.
WT 129/sv mice were treated with vehicle (20% hydroxypropyl β-cyclodextrin, Sigma) for 2.5 days followed by 30 mg/kg PF670462 subcutaneously (Santa Cruz Biotechnology) for 2.5 days. These treatments were administered every 12 h at noon and midnight, as previously described (24). Kidneys were harvested from control and treated mice at midnight, 12 h after the last injection (n = 4 mice/group). Total RNA was isolated from the renal cortex or medulla using TRIzol and converted to cDNA. Real-time quantitative RT-PCR was performed using Taqman primer/probe sets (ABI), as previously described (20). Data were analyzed by an unpaired t-test.
Experiment 4: casein kinase inhibitor treatment for BP experiments.
A separate set of WT 129/sv mice, implanted with radiotelemetry probes, were treated with vehicle for 2.5 days and then with PF670462 for 2.5 days. BP recordings were made during the vehicle treatment period and continued through the 2.5 days of the casein kinase-1δ/ε (CK1δ/ε) inhibitor (CKinh) PF670462 treatment (n = 8 mice in total, allowing for paired t-test comparisons between vehicle and CKinh treatment).
Culture and siRNA Transfection of Xenopus 2F3 Renal Epithelial Cells
Xenopus 2f3 cells (derived from A6 cells) are a kidney epithelial cell line representing the distal nephron. These cells, obtained from the late Dr. Dale Benos, were seeded onto 12-mm permeable transwell inserts (Corning, Tewksbury, MA) and maintained in a 50/50 mixture of DMEM/Ham's F-12 base media (Invitrogen, Grand Island. NY) at pH 7.4 containing 5% FBS (Invitrogen), 1.5 μM aldosterone, 1% streptomycin (GIBCO, Grand Island, NY), and 0.6% penicillin (GIBCO). Cells were kept in a humidified incubator with 4% CO2 in air and at 26°C. Only cells with a passage number of <100 were used for experiments. These standard conditions have been reported in our previously published work (11, 16). 2F3 cells were transiently transfected with either control siRNA or siRNA targeting Xenopus Per1 using Xfect transfection reagent (Clontech, Mountain View, CA) according to the manufacturer’s instructions but with the following modifications. For each 12-well 12-mm permeable transwell insert, 2 μl of 40 μM control siRNA or Xenopus Per1-specific siRNA were incubated with reaction buffer in one microcentrifuge tube while 2 μl of transfection reagent were incubated with reaction buffer in a second microcentrifuge tube. The contents from the latter tube were added to the tube containing siRNA and reaction buffer before the mixture was incubated at room temperature for 10 min. The reaction was added dropwise to the apical side of the cells in a volume of 250 μl of complete growth media. Cells were incubated at 26°C for 6 h before the siRNA transfection solution was replaced with 0.5 ml of complete growth media on the apical side and 1 ml of complete growth media on the basolateral side. Cells were transfected again 48 h later by the same procedure.
Cell-Attached Patch-Clamp Recordings
Micropipettes with a resistance of 6–10 MΩ were made using filamented borosilicate glass capillaries (TW-150F, World Precision Instruments, Sarasota, FL) and a two-stage vertical puller (Narishige, Tokyo, Japan). Distal tubule Xenopus 2F3 cells were subcultured on 12-mm permeable transwell inserts. Cells transfected with either control siRNA or Xenopus Per1-specific siRNA with fluorescein conjugation were selected for using fluorescence. The pipette tip was placed on the cell surface, and negative pressure was applied using a syringe to achieve 10- to 20-GΩ seal resistance. The extracellular bath and patch pipette solutions consisted of physiological saline solution at pH 7.4 and the following (in mM): 95 NaCl, 10 HEPES, 3.4 KCl, 0.8 CaCl2, and 0.8 MgCl2. Different voltages were applied to plot a current-voltage curve. ENaC activity in a patch was calculated using pCLAMP software (Molecular Devices, Sunnyvale, CA) and given as the number of channels [channel density (N)] times the open probability (Po) of the channel (NPo). Results from control siRNA and Xenopus Per1 siRNA-transfected cells were compared by an unpaired t-test.
RESULTS
Loss of Per1 Reduces BP in Salt-Sensitive 129/sv Mice
Previously, we demonstrated that global loss of Per1 in 129/sv mice was protective from the hypertension observed in these mice at baseline (27). To further assess this phenotype, we treated WT and Per1 KO 129/sv mice with a high-salt diet (1% NaCl) and the mineralocorticoid DOCP. This low-renin/high-aldosterone model approximates a state often observed in human essential hypertension (7, 15). In our previously published study (27), we found that global Per1 KO 129/sv mice had a mean arterial pressure (MAP) that was 18 mmHg less than that of WT mice (115 vs. 133 mmHg, P < 0.05). Relative to these baseline MAP values (27), BP was increased in both WT and Per1 KO mice in response to a high-salt diet plus DOCP, and these increases were evident during the night and during the day. After the high-salt diet plus DOCP treatment, global Per1 KO mice on the 129/sv background still exhibit lower MAP compared with WT mice (Fig. 1). WT 129/sv mice exhibited a night/day MAP of 143/134 mmHg, whereas Per1 KO mice had a night/day MAP of 131/123 mmHg (P < 0.001 for night/day effect; P < 0.001 for genotype effect). Thus, loss of Per1 is associated with lower BP despite high-salt diet/DOCP treatment in male 129/sv mice. This result stands in contrast to the nondipping hypertension that we observed in global Per1 KO mice on a C57BL/6 background under similar conditions but with a higher-salt diet (4% NaCl) (25).
Fig. 1.
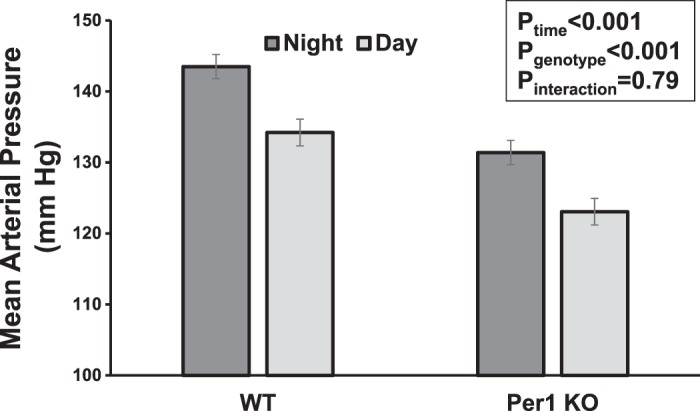
Global Period 1 (Per1) knockout (KO) mice on a 129/sv background exhibit lower blood pressure than wild-type (WT) mice in response to high-salt diet plus desoxycorticosterone pivalate (DOCP). Mice described in our previous study (27) were given a high-salt (1% NaCl) diet followed by DOCP injection. Radiotelemetery recordings of mean arterial pressure were made for 72 h, and the averaged night (active period, 6 PM–6 AM) and day (rest period, 6 AM–6 PM) values are shown. Data were analyzed by two-way ANOVA; n = 6 WT mice and 5 KO mice.
ENaC Contributes to Hypertension in WT 129/sv Mice
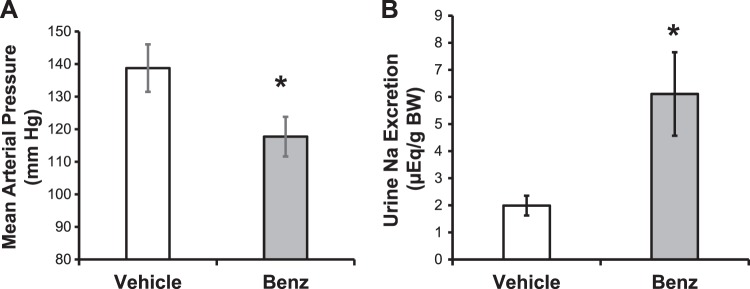
Next, to determine if ENaC contributes to the hypertension observed at baseline in 129/sv mice, we measured BP in conscious, unrestrained mice at the same time that we collected urine as part of a natriuresis assay. We used a novel telemetry receiver/metabolic cage setup (see Ref. 14) to enable urine collection at the same time that BP recordings were made. Benzamil was administrated at 8 PM, 2 h into the active period of these mice, which better approximates the use of diuretics in humans, which are normally taken during their active period. Urine was collected for 4 h, and BP was measured at the same time in the same mice. As shown in Fig. 2, benzamil treatment resulted in a significant decrease in BP (Fig. 2A) and a concomitant increase in urinary Na+ excretion (Fig. 2B).
Fig. 2.
The epithelial Na+ channel (ENaC) contributes to the hypertension observed in wild-type (WT) 129/sv mice on a normal diet. WT 129/sv male mice were placed in metabolic cages that were carefully spaced out to allow for telemetry recordings to be made at the same time as urine collections. Mice were treated with vehicle at 8 PM, and blood pressure (A) and urine Na+ excretion (B) were monitored for 4 h. At 8 PM the following day, the same mice were treated with benzamil and the same measurements were made. BW, body weight. Data are presented as means ± SE; n = 4 mice. *P < 0.05 by a paired t-test.
Pharmacological Inhibition of CK1δ/ε Recapitulates the Effect of Per1 Loss
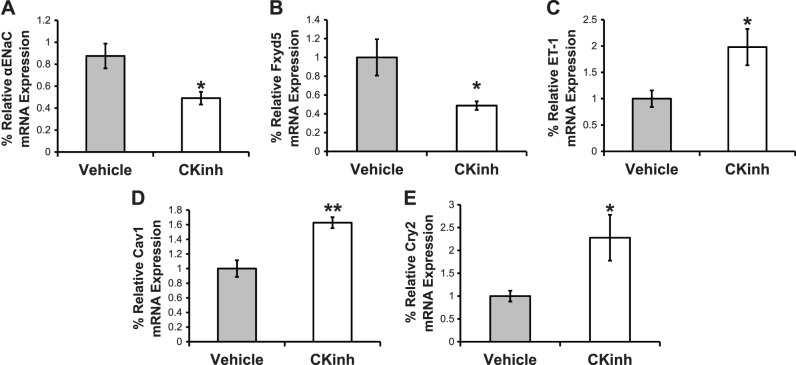
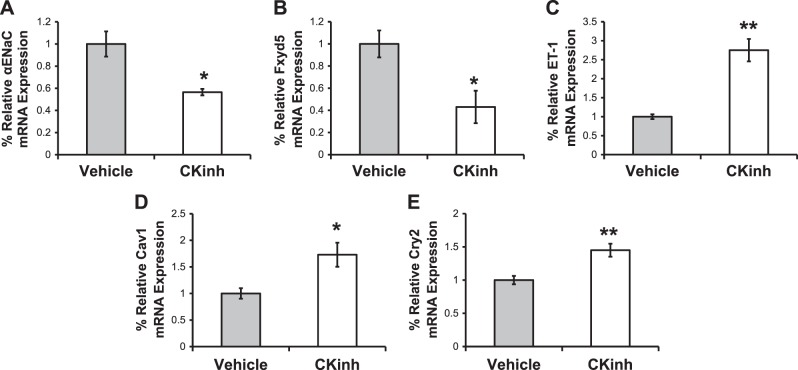
PF670462 (CKinh) inhibits CK1δ/ε, the kinases responsible for phosphorylation of Per1 to allow its subsequent nuclear entry. Previously, we have shown that this inhibitor decreased ENaC activity in vitro (22). In the present study, we treated mice with PF670462 every 12 h, as previously described (20), to ascertain the effect of this inhibitor on Per1 target gene expression in the kidney. We have previously shown that this dose and treatment given to male WT 129/sv mice are associated with decreased levels of nuclear Per1 in the kidney as well as increased Cry2 expression in the renal cortex (24). In the present study, we tested the effect of this treatment on αENaC and three of its regulatory genes, Fxyd5, endothelin-1 (ET-1), and caveolin-1 (Cav-1), in the renal cortex using quantitative PCR. Cry2 expression was increased in the kidneys of treated mice compared with control mice, as expected. Consistent with our previous findings, CKinh treatment was associated with decreased expression of αENaC and Fxyd5 but increased expression of ET-1, Cav-1, and Cry2 (Fig. 3, A–E). Similar results were observed in the renal medulla (Fig. 4, A–E). Thus, CK1δ/ε inhibitor treatment recapitulates effects of Per1 knockdown on gene expression.
Fig. 3.
Period 1 (Per1) target gene expression is altered by casein kinase 1δ/ε (CK1δ/ε) inhibitor (CKinh) treatment in vivo in the renal cortex. After 72 h of treatment with vehicle or CKinh PF670462, kidney tissue was harvested and processed for RNA and quantitative PCR to evaluate changes in mRNA expression for the α-subunit of the epithelial Na+ channel (αENaC; A), Fxyd5 (B), endothelin-1 (ET-1; C), caveolin-1 (Cav1; D), and Cryptochrome 2 (Cry2; E). Data are presented as means ± SE; n = 4 mice/group. Data were analyzed by an unpaired t-test. *P < 0.05; **P < 0.01.
Fig. 4.
Period 1 (Per1) target gene expression is altered by casein kinase 1δ/ε (CK1δ/ε) inhibitor (CKinh) treatment in vivo in the renal medulla. After 72 h of treatment with vehicle or CKinh PF670462, kidney tissue was harvested and processed for RNA and quantitative PCR to evaluate changes in mRNA expression for the α-subunit of the epithelial Na+ channel (αENaC; A), Fxyd5 (B), endothelin-1 (ET-1; C), caveolin-1 (Cav1; D), and Cryptochrome 2 (Cry2; E). Data are presented as means ± SE; n = 4 mice/group. Data were analyzed by an unpaired t-test. *P < 0.05; **P < 0.01.
Inhibition of CK1δ/ε Reduces BP in 129/ sv Mice
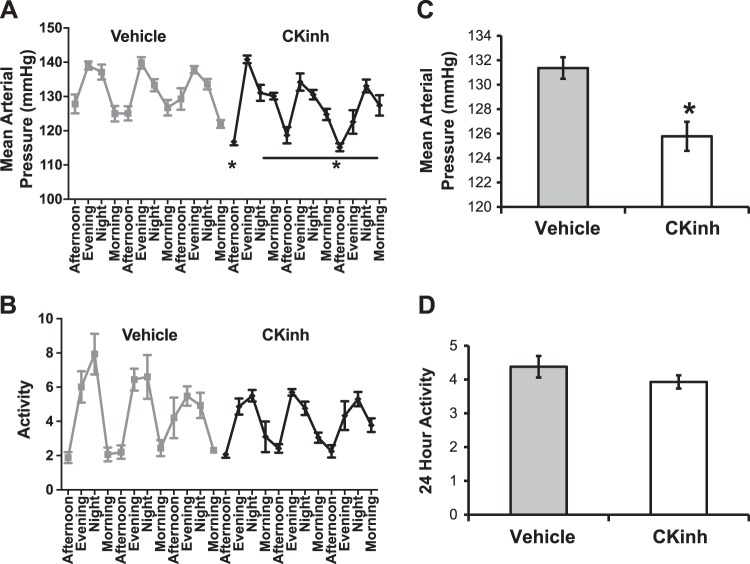
To determine if CKinh treatment recapitulates the effect of Per1 loss on BP, we measured MAP in WT 129/sv mice treated with vehicle or PF670462. Neither vehicle nor CKinh treatment altered the apparent circadian rhythm of BP (Fig. 5A). Overall, 24-h MAP was reduced ~5 mmHg by PF670462 treatment (Fig. 5B). This was not associated with changes in activity rhythms or overall activity (Fig. 5, C and D).
Fig. 5.
Casein kinase 1δ/ε (CK1δ/ε) inhibitor (CKinh) treatment lowers blood pressure in hypertensive 129/sv wild-type mice. Mice implanted with radiotelemetry devices were injected every 12 h with PF670462 (CKinh) at noon and midnight. A and C: blood pressure (A) and activity (C) were monitored continuously. B and D: the average over 72 h showed that mean arterial pressure (B) was significantly reduced with CKinh but activity levels (D) were not affected. Data are presented as means ± SE; n = 8 mice. Data were analyzed by a paired t-test between CKinh and vehicle treatment. *P < 0.05.
Direct Inhibition of Per1 Reduces ENaC Activity
Previously, we demonstrated that Per1 positively regulates the transcriptional activation of the renal αENaC promoter (8). In addition to regulating αENaC at the transcriptional level, we also showed that Per1 regulates ENaC activity in amphibian kidney cells using indirect inhibition of Per1 via PF670462 treatment (22). To further investigate the direct effects of Per1 on ENaC activity in cells representing the distal nephron, we obtained siRNA against Xenopus Per1. Per1 siRNA or scrambled siRNA was mixed with fluorescein-labeled siRNA and transfected into Xenopus 2F3 cells. Fluorescent cells were patched for ENaC activity by the cell-attached single channel patch-clamp technique. The effect of Per1 knockdown on ENaC activity in cultured distal tubule cells is shown in Fig. 6. ENaC activity decreased in cells transfected with Per1 siRNA compared with cells transfected with control siRNA (Fig. 6A). Figure 6, B and C, shows ENaC activity decreased at the level of NPo. Representative single channel recordings showed a decrease in the number of ENaC in the open conformation within a patch for cells transfected with Per1 siRNA compared with cells in the control group (Fig. 6D).
Fig. 6.
Genetic knockdown of Period 1 (Per1) reduces epithelial Na+ channel (ENaC) activity at the single channel level in Xenopus 2F3 distal nephron cells. 2F3 cells were transfected with fluorescein-labeled siRNA directed against Per1 (siPer1) or scrambled control (Con). ENaC activity was assessed by a cell-attached single channel patch-clamp technique. A: number of channels [channel density (N)] times the open probability (Po) of the channel (NPo). B: N. C: Po. The number of recordings made is indicated above each bar. D: representative tracing from a control siRNA-transfected cell compared with a siPer1-transfected cell. C, closed; O, open. Data are presented as means ± SE. Data were analyzed by an unpaired t-test. *P < 0.05; ***P < 0.01.
DISCUSSION
Using genetic and pharmacological inhibition of Per1, we showed that ENaC activity is reduced in cultured kidney cells and that loss or inhibition of Per1 is associated with decreased BP in 129/sv male mice. The results of the present study clearly demonstrate a key role for Per1 in the regulation of ENaC activity as well as BP regulation in 129/sv mice.
Consistent with the role of circadian clock proteins as transcription factors, our results demonstrate that the effect of Per1 on ENaC and BP is associated with changes in expression of several key genes related to Na+ transport mechanisms. Previously, we used genetic knockdown to demonstrate that Per1 positively regulates the expression of genes encoding products that promote renal Na+ reabsorption and negatively regulates the expression of genes encoding products that inhibit renal Na+ reabsorption (27). In the present study, we indirectly inhibited Per1 using pharmacological blockade of Per1 nuclear entry and demonstrated changes in gene expression that are entirely consistent with this model in which Per1 coordinately regulates renal Na+ transport genes. Specifically, αENaC and Fxyd5 mRNA expression increased in response to the CK1δ/ε inhibitor, whereas ET-1 and Cav-1 mRNA expression decreased. Thus, genetic knockdown and pharmacological inhibition of Per1 generate complimentary results.
Consistent with our 2012 finding that global KO of Per1 on the 129/sv background strain resulted in a decrease in BP (27), here we showed that loss of Per1 on this background strain is also associated with lower BP in the setting of a salt- and mineralocorticoid-sensitive model of BP regulation. Using the complimentary approach of pharmacological and indirect inhibition of Per1 in 129/sv mice, we showed a reduction in BP in response to CK1δ/ε inhibition. Together with our previous results (27), the results of the present study suggest that loss of Per1 is protective in the setting of spontaneous hypertension in 129/sv mice that are hypertensive at baseline. It should be noted that this role for Per1 is specific to the 129/sv strain of mice. In contrast, we recently found that global KO of Per1 on the C57BL/6 background strain causes modest changes in BP at baseline but causes nondipping hypertension in response to high-salt diet/DOCP treatment (25). Importantly, these apparent conflicting results are not without precedent since there are known strain differences in BP regulation among several strains of laboratory mouse models (4, 9, 10). The 129/sv mouse model expresses an extra copy of renin, the Ren2 gene, but the contribution of this expression to the BP phenotype of 129/sv mice is not completely understood. Another factor that is likely to contribute to these background strain-dependent differences in cardiovascular function is the extensive genetic variation between 129/sv and C57BL/6 mice (17). Regardless of the underlying mechanism, these stark differences in phenotype between a relatively salt-resistant C57BL/6 strain and the salt-sensitive 129/sv strain may hold important clues for our understanding of BP regulation in genetically distinct human populations, with implications for personalized medicine in the treatment of hypertension.
We previously used the CK1δ/ε inhibitor to show that indirect inhibition of Per1 caused a decrease in ENaC activity in cultured renal epithelial cells (22); in the present study, we used genetic knockdown of Per1 to test the effect on ENaC activity. Here, we showed an effect of direct genetic inhibition of Per1 on ENaC activity in cultured renal epithelial cells. ENaC activity decreased in distal tubule cells transfected with Per1 siRNA compared with cells transfected with control siRNA. These studies suggest that Per1 regulation of ENaC extends beyond the transcriptional level and that Per1 knockdown attenuates ENaC density and Po at the apical plasma membrane of distal tubule cells. Future studies should be performed to investigate whether Per1 indirectly regulates ENaC protein expression and activity at the apical plasma membrane by regulating chaperone proteins involved in its insertion into the membrane and effector proteins involved in its degradation and recycling back to the membrane. Moreover, studies should be performed to determine whether Per1 regulates actin cytoskeleton proteins such as myristoylated alanine-rich protein kinase C substrate and filamin, which have been shown to play an essential role in stabilizing ENaC at the membrane (1, 2, 19, 31).
The results of the present study are entirely consistent with our previous work in several different renal cell lines demonstrating a role for Per1 in the positive regulation of renal Na+ reabsorption (22–24, 26). Moreover, these results also fit with the role of Per1 as protective in BP regulation in the 129/sv mouse (27). It is tempting to speculate that renin2-expressing 129/sv mice may share a steroid-rich milieu with the typical high-aldosterone conditions used in cultured kidney cells. A limitation of the present study is that the animal experiments were limited to male mice on the 129/sv background strain. Female Per1 KO mice on the 129/sv background have not been studied in terms of renal function or BP. We recently demonstrated that female Per1 KO mice on the C57Bl/6 background do not exhibit a BP phenotype under control conditions or in response to high-salt diet/DOCP (6).
In summary, we used complimentary genetic and pharmacological inhibition to inhibit the action of Per1 directly and indirectly to demonstrate a key role for this circadian clock protein in the regulation of ENaC and BP. These results support an important link between renal Na+ handling and the molecular clock and may help connect two key homeostatic mechanisms: the renin-angiotensin-aldosterone system that tightly regulates renal Na+ reabsorption and the circadian clock that tightly regulates tissue-specific physiological function.
GRANTS
This work was supported by the University of Florida College of Medicine and National Institute of Diabetes and Digestive and Kidney Diseases Grants K01-DK-099617 (to A. Alli) and R01-DK-109570 and R03-DK-098460 and the American Society for Nephrology Foundation for Research (to M. L. Gumz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A. and M.L.G. conceived and designed research; A.A., L.Y., J.R., K.-Y.C., I.J.L., and M.L.G. performed experiments; A.A., L.Y., M.H., J.R., K.-Y.C., I.J.L., and M.L.G. analyzed data; A.A., L.Y., J.R., I.J.L., C.S.W., and M.L.G. interpreted results of experiments; A.A., L.Y., M.H., J.R., and M.L.G. prepared figures; A.A., L.Y., and M.L.G. drafted manuscript; A.A., L.Y., M.H., I.J.L., C.S.W., and M.L.G. edited and revised manuscript; A.A., L.Y., and M.L.G. approved final version of manuscript.
REFERENCES
- 1.Alli AA, Bao HF, Alli AA, Aldrugh Y, Song JZ, Ma HP, Yu L, Al-Khalili O, Eaton DC. Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am J Physiol Renal Physiol 303: F800–F811, 2012. doi: 10.1152/ajprenal.00703.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alli AA, Bao HF, Liu BC, Yu L, Aldrugh S, Montgomery DS, Ma HP, Eaton DC. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am J Physiol Renal Physiol 309: F456–F463, 2015. doi: 10.1152/ajprenal.00631.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman JM, Mironova E, Stockand JD. Physiological regulation of the epithelial Na+ channel by casein kinase II. Am J Physiol Renal Physiol 314: F367–F372, 2018. doi: 10.1152/ajprenal.00469.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang J, Ma JZ, Zeng Q, Cechova S, Gantz A, Nievergelt C, O’Connor D, Lipkowitz M, Le TH. Loss of GSTM1, a NRF2 target, is associated with accelerated progression of hypertensive kidney disease in the African American Study of Kidney Disease (AASK). Am J Physiol Renal Physiol 304: F348–F355, 2013. doi: 10.1152/ajprenal.00568.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med 119: 108–114, 2018. doi: 10.1016/j.freeradbiomed.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douma LG, Solocinski K, Holzworth MR, Crislip GR, Masten SH, Miller AH, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Female C57BL/6J mice lacking the circadian clock protein PER1 are protected from nondipping hypertension. Am J Physiol Regul Integr Comp Physiol 316: R50–R58, 2019. doi: 10.1152/ajpregu.00381.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durukan M, Guray U, Aksu T, Guray Y, Demirkan B, Korkmaz S. Low plasma renin activity and high aldosterone/renin ratio are associated with untreated isolated systolic hypertension. Blood Press 21: 320–325, 2012. doi: 10.3109/08037051.2012.686167. [DOI] [PubMed] [Google Scholar]
- 8.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurley SB, Mach CL, Stegbauer J, Yang J, Snow KP, Hu A, Meyer TW, Coffman TM. Influence of genetic background on albuminuria and kidney injury in Ins2(+/C96Y) (Akita) mice. Am J Physiol Renal Physiol 298: F788–F795, 2010. doi: 10.1152/ajprenal.90515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartner A, Cordasic N, Klanke B, Veelken R, Hilgers KF. Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol Dial Transplant 18: 1999–2004, 2003. doi: 10.1093/ndt/gfg299. [DOI] [PubMed] [Google Scholar]
- 11.Jella KK, Yu L, Yue Q, Friedman D, Duke BJ, Alli AA. Exosomal GAPDH from proximal tubule cells regulate ENaC activity. PLoS One 11: e0165763, 2016. doi: 10.1371/journal.pone.0165763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleyman TR, Kashlan OB, Hughey RP. Epithelial Na+ channel regulation by extracellular and intracellular factors. Annu Rev Physiol 80: 263–281, 2018. doi: 10.1146/annurev-physiol-021317-121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, Yang X, Mo X, Huang J, Chen J, Kelly TN, Hixson JE, Rao DC, Gu CC, Shimmin LC, Chen J, Rice TK, Li J, Schwander K, He J, Liu DP, Gu D. Associations of epithelial sodium channel genes with blood pressure: the GenSalt study. J Hum Hypertens 29: 224–228, 2015. doi: 10.1038/jhh.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch IJ, Welch AK, Gumz ML, Kohan DE, Cain BD, Wingo CS. Effect of mineralocorticoid treatment in mice with collecting duct-specific knockout of endothelin-1. Am J Physiol Renal Physiol 309: F1026–F1034, 2015. doi: 10.1152/ajprenal.00220.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meneton P, Galan P, Bertrais S, Heudes D, Hercberg S, Ménard J. High plasma aldosterone and low renin predict blood pressure increase and hypertension in middle-aged Caucasian populations. J Hum Hypertens 22: 550–558, 2008. doi: 10.1038/jhh.2008.27. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery DS, Yu L, Ghazi ZM, Thai TL, Al-Khalili O, Ma HP, Eaton DC, Alli AA. ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins. Am J Physiol Cell Physiol 313: C42–C53, 2017. doi: 10.1152/ajpcell.00244.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res 14: 1806–1811, 2004. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray EC, Chen J, Kelly TN, He J, Hamm LL, Gu D, Shimmin LC, Hixson JE, Rao DC, Sheng S, Kleyman TR. Human epithelial Na+ channel missense variants identified in the GenSalt study alter channel activity. Am J Physiol Renal Physiol 311: F908–F914, 2016. doi: 10.1152/ajprenal.00426.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reifenberger MS, Yu L, Bao HF, Duke BJ, Liu BC, Ma HP, Alli AA, Eaton DC, Alli AA. Cytochalasin E alters the cytoskeleton and decreases ENaC activity in Xenopus 2F3 cells. Am J Physiol Renal Physiol 307: F86–F95, 2014. doi: 10.1152/ajprenal.00251.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards J, All S, Skopis G, Cheng KY, Compton B, Srialluri N, Stow L, Jeffers LA, Gumz ML. Opposing actions of Per1 and Cry2 in the regulation of Per1 target gene expression in the liver and kidney. Am J Physiol Regul Integr Comp Physiol 305: R735–R747, 2013. doi: 10.1152/ajpregu.00195.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards J, Cheng KY, All S, Skopis G, Jeffers L, Lynch IJ, Wingo CS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am J Physiol Renal Physiol 305: F1697–F1704, 2013. doi: 10.1152/ajprenal.00472.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards J, Greenlee MM, Jeffers LA, Cheng KY, Guo L, Eaton DC, Gumz ML. Inhibition of αENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CK1δ/ε. Am J Physiol Renal Physiol 303: F918–F927, 2012. doi: 10.1152/ajprenal.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards J, Jeffers LA, All SC, Cheng KY, Gumz ML. Role of Per1 and the mineralocorticoid receptor in the coordinate regulation of αENaC in renal cortical collecting duct cells. Front Physiol 4: 253, 2013. doi: 10.3389/fphys.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards J, Ko B, All S, Cheng KY, Hoover RS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem 289: 11791–11806, 2014. doi: 10.1074/jbc.M113.531095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol (Oxf) 220: 72–82, 2017. doi: 10.1111/apha.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solocinski K, Richards J, All S, Cheng KY, Khundmiri SJ, Gumz ML. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Renal Physiol 309: F933–F942, 2015. doi: 10.1152/ajprenal.00197.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59: 1151–1156, 2012. doi: 10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. J Clin Invest 128: 2157–2167, 2018. doi: 10.1172/JCI80590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiwari S, Nordquist L, Halagappa VK, Ecelbarger CA. Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol 293: F178–F185, 2007. doi: 10.1152/ajprenal.00447.2006. [DOI] [PubMed] [Google Scholar]
- 30.Van Laake LW, Luscher TF, Young ME. The circadian clock in cardiovascular regulation and disease: lessons from the Nobel Prize in Physiology or Medicine 2017. Eur Heart J 39: 2326–2329, 2018. doi: 10.1093/eurheartj/ehx775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q, Dai XQ, Li Q, Tuli J, Liang G, Li SS, Chen XZ. Filamin interacts with epithelial sodium channel and inhibits its channel function. J Biol Chem 288: 264–273, 2013. doi: 10.1074/jbc.M112.396408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111: 16219–16224, 2014. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Li L, Kohan DE, Ecelbarger CM, Kishore BK. Attenuation of lithium-induced natriuresis and kaliuresis in P2Y2 receptor knockout mice. Am J Physiol Renal Physiol 305: F407–F416, 2013. doi: 10.1152/ajprenal.00464.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]