Fig. 1.
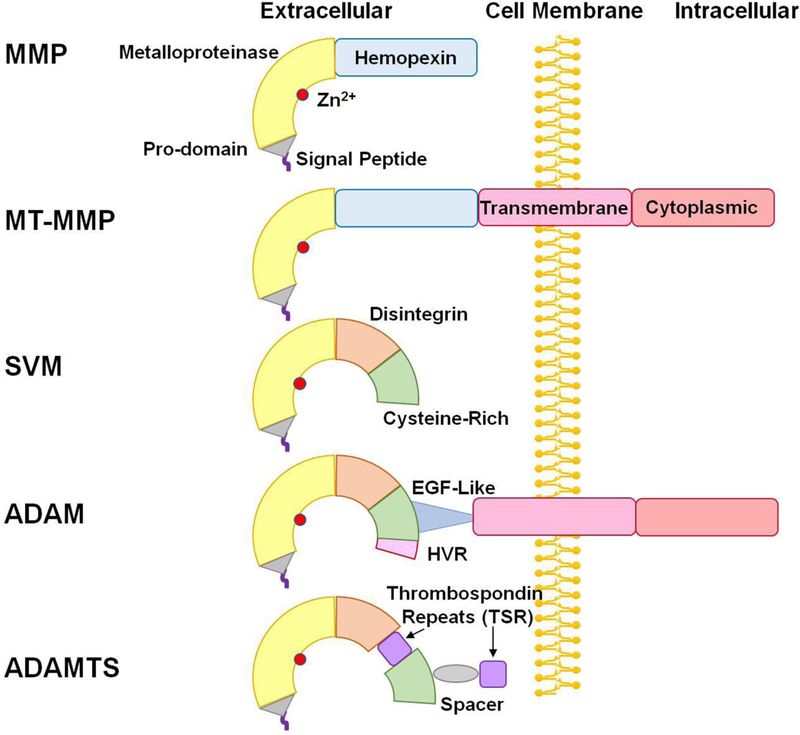
Structure of ADAM and ADAMTS. In addition to the signal peptide, pro-domain, and metalloproteinase domain in matrix metalloproteinases (MMP) and membrane-type MMPs (MT-MMP), snake venom metalloproteinases (SVM) have a disintegrin and cysteine-rich domain. Compared with SVM, ADAMs have a highly variable region (HVR) in the cysteine-rich domain, and an additional epidermal growth factor (EGF)-like region, transmembrane domain and cytoplasmic tail. ADAMTS members are similar to ADAMs, but lack the EGF-like region, transmembrane domain and cytoplasmic tail, and instead have thrombospondin repeats (TSR) and spacer.
