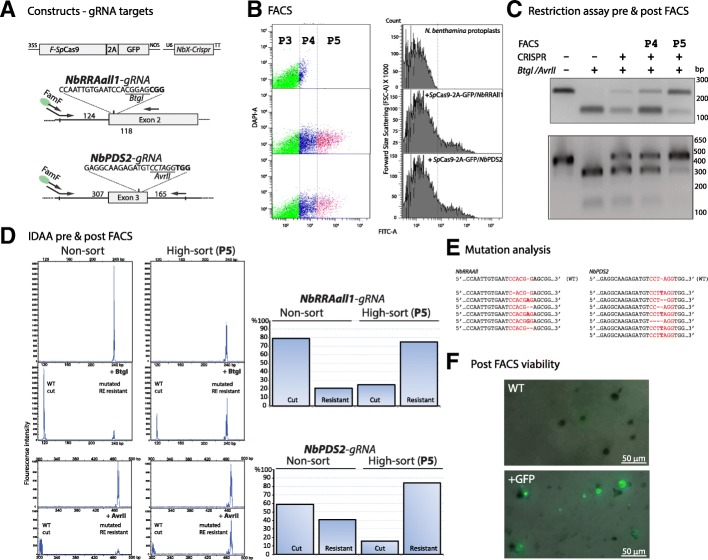
Fig. 3.
FACS mediated enrichment of gRNA/SpCas9 expressing protoplast cells and resulting mutations. 3–5 N. benthamiana leaves were infiltrated with Agrobacterium-delivered replicons expressing SpCas9-2A-GFP together with NbRRAall-gRNA or NbPDS2-gRNA (a), respectively, and left for 2–4 days. b WT protoplasts and protoplasts expressing SpCas9-2A-GFP and NbRRAall1- or NbPDS2-gRNA were subjected to GFP mediated FACS. The DAPI and FITC intensities for protoplasts were recorded and three populations, P3, P4 & P5, with the P3 population corresponding to non-transformed cells and the P4 and P5 populations representing intermediary and high stringently sorted cell populations, respectively, were selected from the Dot Scattering Chromatograms. Transfected protoplasts were defined as FITC-positive events and gates were set to separate WT and GFP enriched protoplast populations, using the WT sample to define non-transfected wild type populations (P3) in the transfected samples. P4 and P5 (GFP enriched populations) were gated with medium and high FITC signal intensity. c RE analysis of PCR amplified target regions using BtgI and AvrII for NbRRAall1- and NbPDS2 gRNAs, respectively, demonstrating indel formation in unsorted and indel enrichment in FACS sorted (P4 and P5) populations. Indel enrichment in P5 populations were cooperated by the IDAA technique (d) where the additional restriction enzyme digest allows for visualization of the mutated population without the presence of non-mutated PCR amplicons (‘mutated / RE resistant’ designates the RE site were mutated rendering it resistant for digestion while ‘WT/cut’ designates WT sites that were cut and moved downstream in the chromatogram). e Sequence analysis of RE-resistant PCR fragments of the two P5 populations. f Post viability of protoplasts was assessed in WT protoplasts (dark circular objects) without detectable GFP signal and GFP fluorescence in Cas9-2A-GFP sorted protoplasts (presented as an overlay of light and fluorescent micrographs). FACS was carried out using a FACSAria III (BD Biosciences) apparatus with procedure and parameters as outlined in the Methods section and IDDA as described in [39]. For the viability test shown in f crude protoplasts were prepared and sorted on a Sony Cell sorter SH800S with sorting gating parameters similar to those used on the BD FACSAria III sorter and with the W5 buffer as recipient buffer