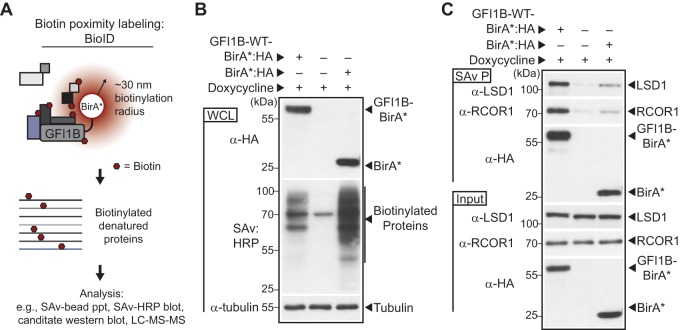
FIG 3.
Proximity labeling of GFI1B binding proteins with GFIB-BirA*. (A) Schematic representation of the strategy for biotin modification of GFI1B partners. GFI1B-WT is fused to the promiscuous biotin ligase with an HA epitope tag (BirA*:HA) to form GFI1B-WT-BirA*:HA. GFI1B partners are spontaneously biotinylated due to their proximity to BirA*:HA anchored to GFI1B in cis. Biotinylated products are captured on streptavidin (SAv)-conjugated beads for downstream analysis via candidate-based or screening approaches. (B) BirA*:HA and GFI1B-WT-BirA*:HA fusion biotinylate diverse targets in K562 cells. Cells were transduced with constructs inducibly expressing GFI1B-WT-BirA*:HA, BirA*:HA, or EV, treated with doxycycline, and harvested. GFI1B-WT-BirA*:HA and BirA*:HA expression was confirmed by anti-HA Western blotting and biotinylated proteins detected by SAv:HRP. Tubulin served as a loading control. (C) GFI1B-interacting proteins LSD1 and RCOR1 are enriched among proteins biotinylated by GFI1B-WT-BirA*:HA compared to BirA*:HA or EV. Biotin-modified proteins were collected on SAv-Sepharose. LSD1, RCOR1, and the BirA*:HA fusion proteins themselves were quantified among purified proteins (SAv P) by Western blotting. Equal expression was confirmed by Western blotting in whole-cell lysates (input).