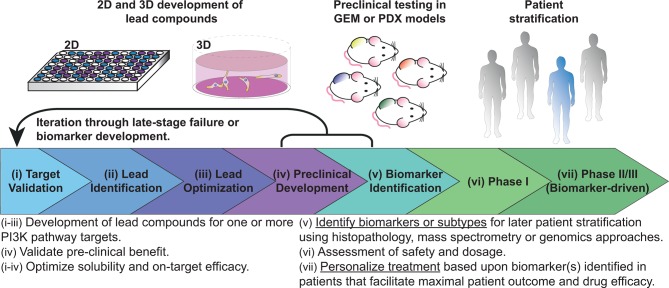
Figure 2.
Adaptable drug development pipeline, demonstrating the progression of lead compounds through target validation, lead compound identification and optimisation, then preclinical validation. The necessary addition to this process is the identification of biomarkers to guide both lead compound development and later stratification in phase II/III clinical trials. These processes may be iterated to improve on-target efficacy, solubility and biomarkers. After safety and tolerability is confirmed in phase I clinical trials, biomarker-driven phase II/III may reduce the high attrition rates of lead compounds if appropriate patient stratification can demonstrate beneficial response in the assessed subsets of patients. These biomarkers may also provide opportunities for retrospective analysis and later iteration into clinical trials. PI3K, phosphoinositide 3-kinase.