FIG 1.
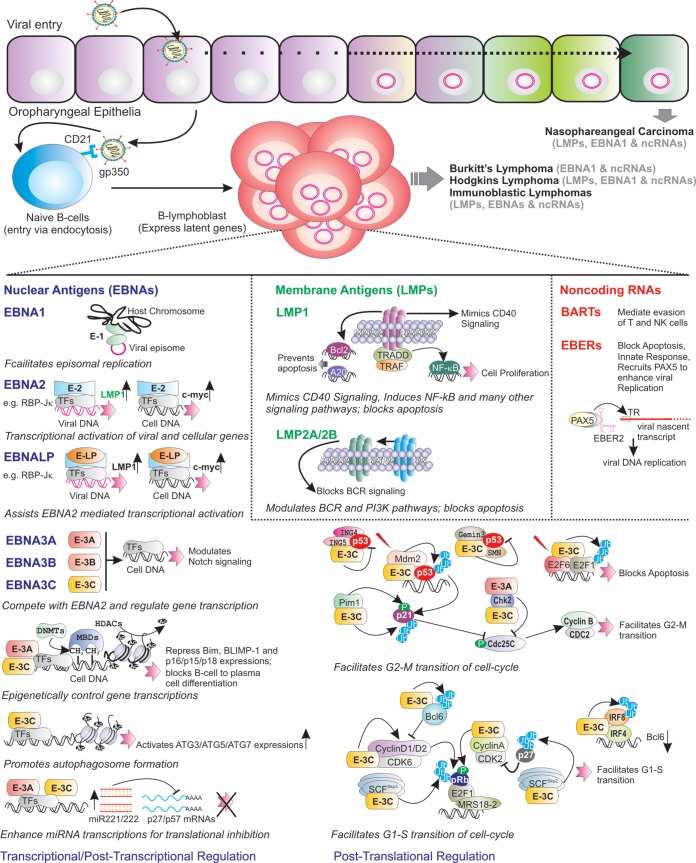
Salient features of EBV latent transcripts during B-cell transformation, followed by B-cell lymphoma development. After initial infection of oropharyngeal epithelial cells, EBV primarily infects the naive B lymphocytes. Subsequently, the infected B cells are growth transformed, expressing a subset of viral genes, with 6 nuclear antigens (EBNAs), 3 membrane proteins (LMPs), and several noncoding RNAs (EBERs and BARTs). EBNA1 binds to the episome origin of replication to allow viral genome replication. EBNA2 transcriptionally activates a number of viral (red) and cellular (black) genes through recruiting cell transcription factors (TFs), like RBP-Jκ, and induces cell growth. EBNALP promotes EBNA2-mediated gene transcription. EBNA3 proteins (EBNA3A, EBNA3B, and EBNA3C) modulate viral gene and Notch signaling by blocking EBNA2 association with RBP-Jκ. Both EBNA3A and EBNA3C recruit several epigenetic modifications (such as polycomb repressor complex 2 [PRC2]) to transcriptionally repress BIM, BLIMP-1, and p15, p16, and p18 expression and inhibit B-cell-to-plasma cell differentiation. Through epigenetic control, EBNA3C transactivates ATG3, ATG5, and ATG7 expression, thereby promoting autophagosome formation. EBNA3A and EBNA3C enhance miR221/222 transcription, which in turn block p27 and p57 translation. EBNA3C employs several mechanisms to block p53-mediated apoptotic activities. For example, EBNA3C recruits Mdm2 E3 ligase activity and stabilizes Gemin3 to enhance p53 degradation, and it competes with ING4 and ING5 binding to block p53-dependent apoptosis. EBNA3C enhances Pim-1-mediated p21 phosphorylation and degradation. Both EBNA3A and EBNA3C interact with Chk2 and facilitate the G2-M transition. In response to DNA damage signals, EBNA3C enhances E2F1 degradation, thereby blocking E2F1-mediated apoptosis. EBNA3C binds to E2F6 to block E2F1-mediated transcription. EBNA3C forms complexes and enhances the kinase activities of CyclinD1/CDK6, CyclinD2/CDK6, and CyclinA/CDK2 and augments pRb phosphorylation. EBNA3C recruits IRF4 to block Bcl6 expression and enhances IRF8 degradation. EBNA3C increases ubiquitin-proteasome-mediated degradation of hyperphosphorylated pRb, p27, and Bcl6, which facilitates the G1-S transition of the cell cycle. LMP1 mimics CD40 signaling and prevents apoptosis by upregulating bcl-2 and A20. LMP1, through interacting with tumor necrosis factor receptor (TNFR)-associated factors (TRAFs) and TNFR-associated death domain (TRADD) protein, constitutively induces NF-κB signaling pathway. LMP1 also activates JAK/STAT, ERK mitogen-activated protein kinase (MAPK), IRF, and Wnt signaling pathways. LMP2A blocks B-cell receptor (BCR) signaling, while LMP2B regulates LMP2A functions. EBV noncoding RNAs, the EBERs (EBER1 and EBER2), regulate the innate immune response and block apoptosis. EBER2 recruits PAX5 to the terminal repeat (TR) region of nascent viral transcript, which helps for viral lytic replication. BARTs mediate the evasion of T and NK cells during infection of B cells in peripheral blood lymphocytes.