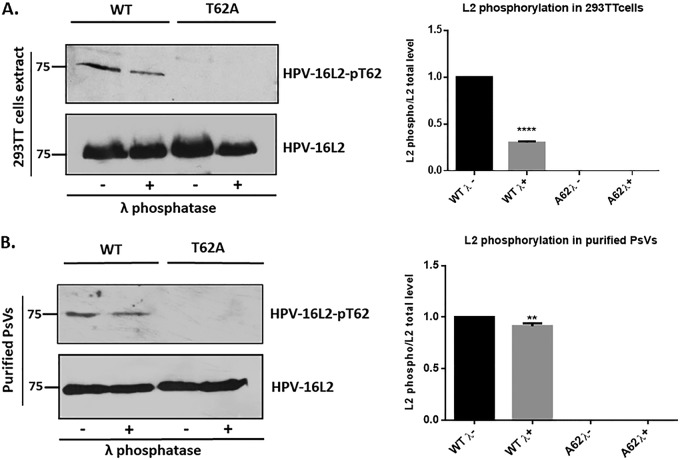
FIG 2.
Confirmation of L2 phosphorylation using a phospho-specific HPV-16 L2-pT62 antibody. (A) HEK293TT cells were transfected with plasmids expressing wild-type (WT) and mutant (T62A) L2. After 24 h the cells were harvested and incubated in the presence (+λ) or absence (−λ) of lambda phosphatase for 30 min. Western blotting was then performed for phosphorylated L2 (top panel) and total L2 (bottom panel). Quantification of phosphorylated L2 normalized to the amount of total L2 is shown in the histogram. Note that wild-type HPV-16 L2 is clearly phosphorylated in HEK293TT cell extracts, with a decrease in signal following treatment with lambda phosphatase, and no phosphorylation is seen with the T62A mutant. (B) The cesium chloride gradient-purified wild-type and mutant (T62A) PsVs were also analyzed by Western blotting using the same phospho-specific HPV-16 L2-pT62 antibody. Purified PsVs were incubated with (+λ) or without (−λ) lambda phosphatase and then analyzed by Western blotting using the anti-HPV-16 L2 phospho-specific antibody and the total anti-L2 antibody. Quantification of phosphorylated L2 normalized to the amount of total L2 is shown in the histogram. **, P < 0.01; ****, P < 0.0001. Note that these results again confirm the presence of a phospho-acceptor site at T62 in the context of purified viral capsids. Taken together, these results demonstrate that the HPV-16 L2 protein is phosphorylated at T62 both in cell extracts and in purified PsVs.