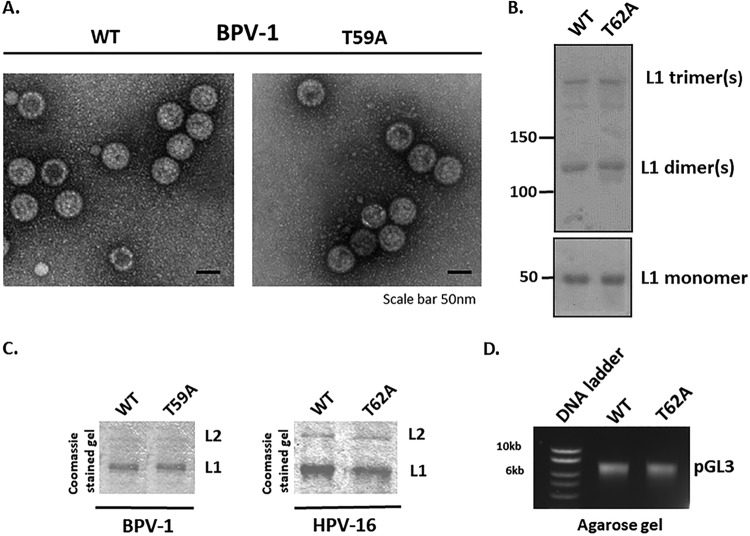
FIG 4.
Mutation of the L2 phospho-acceptor site does not affect PsVs assembly. (A) The integrity of purified wild-type and mutant (T59A) BPV-1 PsVs was monitored by electron microscopic analysis. Note that no evident structural differences were observed between the wild-type and mutant PsV preparations. (B) The patterns of disulfide bonds in the purified wild-type and T62A HPV-16 PsVs were examined by nonreducing denaturing gel analysis and Ponceau S staining (upper panel). The lower panel shows conventional denaturing gel analysis of both PsV preparations. Note that the two PsV preparations show similar levels of disulfide cross-linking, which correspond to those of fully mature capsids. (C) The Coomassie-stained gels of the purified PsVs show similar levels of capsid protein for wild-type and T59A BPV-1 PsVs and for wild-type and T62A HPV-16 PsVs, indicating that coassembly of L1/L2 proteins into particles is not affected by the T62A substitution. (D) The agarose gel shows similar amounts of encapsidated DNA (pGL3 vector carrying the luciferase reporter gene) extracted from the purified wild-type and T62A HPV-16 PsVs.