FIG 5.
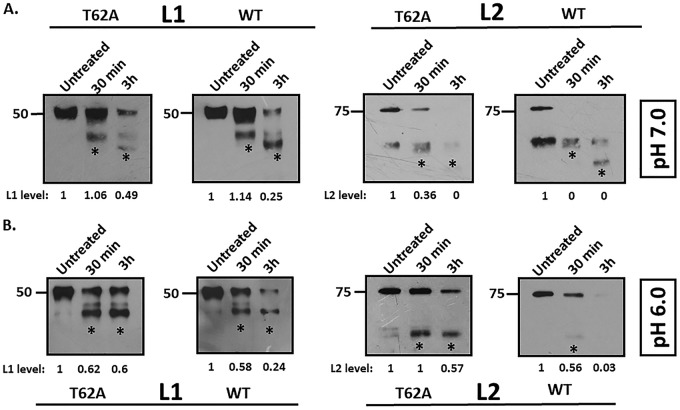
Mutation of the L2 phospho-acceptor site alters PsV stability. (A) Trypsin digestion of HPV-16 PsVs at pH 7.0. Purified T62A and wild-type HPV-16 PsVs were incubated with an equal volume of 0.05% trypsin at pH 7.0 for either 30 min or 3 h and compared with untreated samples by Western blot analysis. Panels show anti-L1 and anti-L2 immunoblots. Note the increased intensity of the lower bands (*, tryptic products) in the trypsin-treated wild-type PsVs. Clearly, the total L2 protein levels are greatly reduced in wild-type preparations in comparison to those in the T62A preparations, with the wild-type L2 protein being almost undetectable after 30 min of trypsin digestion. (B) Trypsin digestion of HPV-16 PsVs at pH 6.0. Purified T62A and wild-type HPV-16 PsVs were incubated with an equal volume of 0.05% trypsin at pH 6.0 for either 30 min or 3 h and compared with untreated samples by Western blot analysis. Panels show anti-L1 and anti-L2 immunoblots. Note that L1 proteins from wild-type and T62A PsVs show similar tryptic products following trypsin treatment at pH 6.0. However, digestion of L1 was much more rapid in the wild-type PsV preparations than in the T62A PsVs. In the case of L2, both the total levels and the tryptic products of wild-type L2 were significantly lower than those of T62A PsVs after 30 min of trypsin digestion at pH 6.0. Taken together, these data show that the T62 residue in the L2 phosphorylation site has a major influence on viral sensitivity to trypsin, indicating that PsVs lacking the T62 phospho-acceptor site are significantly more resistant to trypsin digestion than the wild-type PsVs. For all experiments, total L1 and total L2 protein levels were quantified by measuring the pixel intensity of each band using densitometry (ImageJ) and are shown normalized to levels of their respective (untreated) controls.