FIG 6.
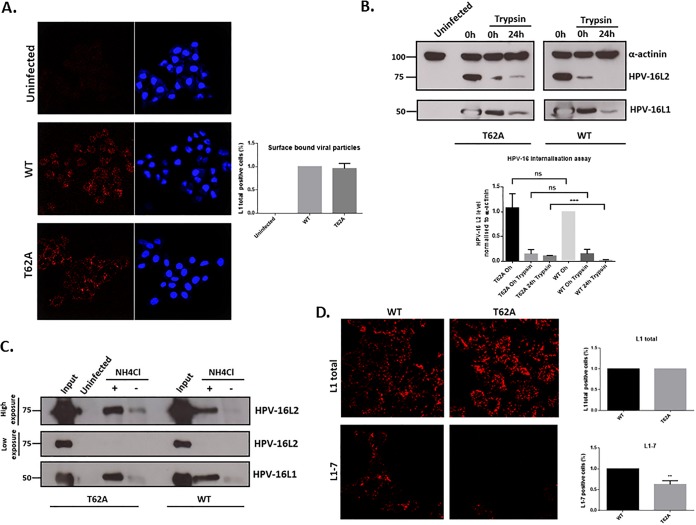
The T62A phospho-acceptor site has no effect on virus internalization but does affect intracellular processing. (A) PsV binding to the cell surface. HeLa cells were exposed to wild-type and T62A HPV-16 PsVs for 24 h. The cells were then washed, fixed, and subjected to quantitative immunofluorescence analysis of nonpermeabilized cells using an L1-specific antibody (red). The nuclei were detected with 4′,6′-diamidino-2-phenylindole (blue). Representative pictures are shown. Quantification of surface-bound viral particles is also shown. Note that similar amounts of wild-type and T62A HPV-16 L1-positive PsVs are observed after L1 staining, confirming that there are no major differences in how both types of PsVs bind to the surface of infected cells. (B) PsV internalization assay. HeLa cells were infected with wild-type and T62A HPV-16 PsVs. At 1 h and 24 h postinfection, the cells were treated with trypsin to remove any noninternalized virus. They were then harvested, and the levels of L1 and L2 protein remaining within the cells were monitored by Western blotting. Quantification of HPV-16 L2 levels, normalized to alpha-actinin, is shown in the histogram. Note that there are similar levels of L1 and L2 in wild-type and T62A PsVs at 1 h postinfection (0 h trypsin), indicating no major differences in levels of PsV entry. Interestingly, the intensity of T62A L2 is much higher than that of wild-type L2 at 24 h postinfection (24 h trypsin), suggesting that the processing of T62A PsVs in HeLa cells is slower than that of the wild-type. (C) Intracellular processing of PsVs. HaCaT cells were exposed to wild-type and T62A PsVs and incubated for 24 h at 37°C. The cells were then removed with 0.25% trypsin, washed twice with PBS, and lysed in RIPA lysis buffer. Clarified lysates were incubated with mouse anti-HPV-16 L1 (H16.V5) antibody followed by protein A-conjugated Sepharose beads. Immunoprecipitated complexes were collected by centrifugation, washed three times, resolved by 10% SDS-PAGE, and analyzed by Western blotting. HPV-16 L1 was detected with the CamVir-1 antibody. For determination of L1-associated L2 protein, mouse anti-HPV-16 L2 (16.D4 64-81) was used. The acidification inhibitor NH4Cl was included as a positive control for L2 retention. Note that in the input capsids, the levels of T62A and wild-type capsid-associated L2 protein were equal. However, in the untreated cells, the T62A HPV-16 PsVs showed a much stronger association between L1 and T62A L2 than with the wild-type L2. When the cells were treated with NH4Cl, increased levels of both T62A and wild-type L2 remained associated with L1 compared to levels with untreated cells; however, there was no major difference in T62A and wild-type L2 levels. These results further indicate a delay in the processing of HPV-16 T62A mutant PsVs compared with that of wild-type virus. (D) Capsid disassembly assay. HeLa cells were infected with wild-type and T62A HPV-16 PsVs. At 7 h postinfection the cells were fixed and stained using mouse anti-L1 antibody (CamVir-1) for total L1 or the uncoating-specific anti-L1 antibody 33L1-7 (red). Representative pictures are shown. Quantification of virus disassembly based on the 33L1-7 staining is also shown. Note that capsid disassembly is approximately 40% lower in the T62A PsV-infected cells than in wild-type-infected cells. These results indicate that the absence of an L2 phospho-acceptor site at T62 increases capsid stability and delays the process of capsid uncoating. **, P < 0.01; ***, P < 0.001.