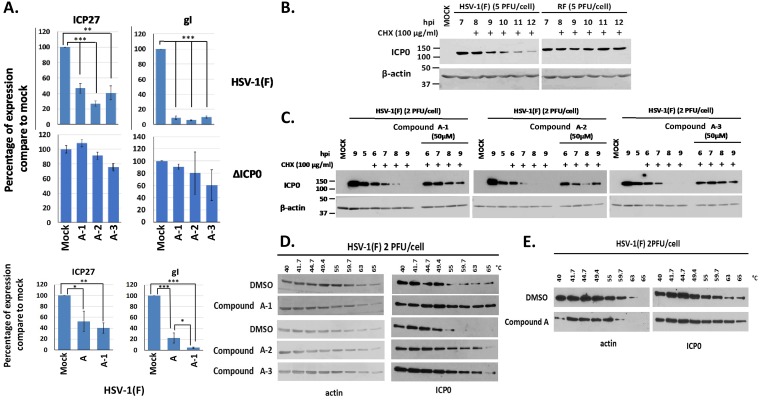
FIG 7.
Effects of the compound A analogues on HSV-1 viral gene expression and the stability of ICP0. (A) HEL cells were infected with HSV-1(F) or ΔICP0 virus (0.1 PFU/cell) and either not treated or treated with compound A, A-1, A-2, or A-3 at 20 μM, added to the cultures at the time of infection. The cells were harvested at 8 h postinfection, and viral gene expression was quantified by qPCR analysis. The 18S rRNA primers were used for normalization. Error bars represent standard deviations. Statistical analysis was performed using unpaired t tests. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. (B) HEp-2 cells were infected with HSV-1(F) or an ICP0 RF mutant virus (5 PFU/cell). CHX (100 μg/ml) was added to the cultures at 7 h postinfection. The cells were harvested at the time of CHX addition or at 1, 2, 3, 4, and 5 h posttreatment, and ICP0 protein was assessed in equal amounts of cell lysates by immunoblot analysis. β-Actin served as a loading control. (C) HEp-2 cells were infected with HSV-1(F) (2 PFU/cell), compound A-1, A-2, or A-3 (50 μM) was added to the infected cultures at 4.5 h postinfection, and CHX (100 μg/ml) was added to the cultures 0.5 h later. The cells were harvested at 0, 1, 2, 3, and 4 h after the addition of CHX, whereas an infected untreated sample harvested at 9 h after infection served as a control. The amounts of ICP0 protein were assessed in equal amounts of cell lysates by immunoblot analysis. β-Actin served as a loading control. (D) HEp-2 cells were infected with HSV-1(F) (2 PFU/cell) for 4 h and incubated with the indicated compounds (50 μM) for an extra 1 h. Cells were then collected and CETSA was performed. ICP0 temperature stability was assessed in the soluble fraction by immunoblot analysis. (E) The experiment was as in panel D except that HEL cells were used and compound A was added to the cultures at 20 μM for 1 h at 16 h postinfection.