Figure 5.
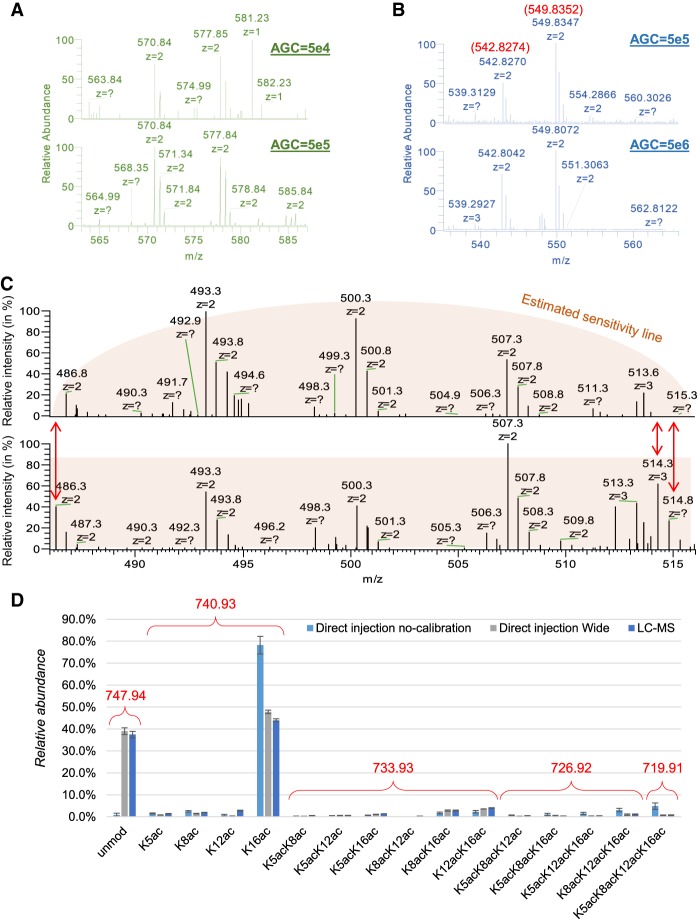
Optimization of the AGC target and acquisition window for DI-MS. (A) Example displaying how an acquisition using 50,000 ions as AGC is less indicated than 500,000 ions to obtain a clear S/N ratio for the two peptides of histone H3 KQLATKAAR (aa 18–26) unmodified (577.85 m/z) and with one acetyl (570.84 m/z). This sample was derivatized at the peptide N-termini. (B) Example showing that 5,000,000 as AGC target creates issues of mass accuracy as compared to 500,000. The peptides are the same as panel A, but they were not derivatized at the N-termini in this experiment. (C) Example of incorrect (top) and correct (bottom) calibration of the quadrupole wide isolation in an Orbitrap Fusion. The signals at the edges of the acquisition window are lost, creating significant biases in the estimation of peptide abundance. (D) Relative abundance of the peptide of histone H4 GKGGKGLGKGGAKR (aa 4–17) detected using uncalibrated quadrupole wide isolation using DI-MS with narrow acquisition, with an extra 2 m/z acquisition on both ends of the acquisition window and by canonical LC-MS. It is evident that, even without calibration, the acquisition is correctly performed using wide windows and that all isobaric forms of this peptide (which contains four modifiable residues) were resolved.