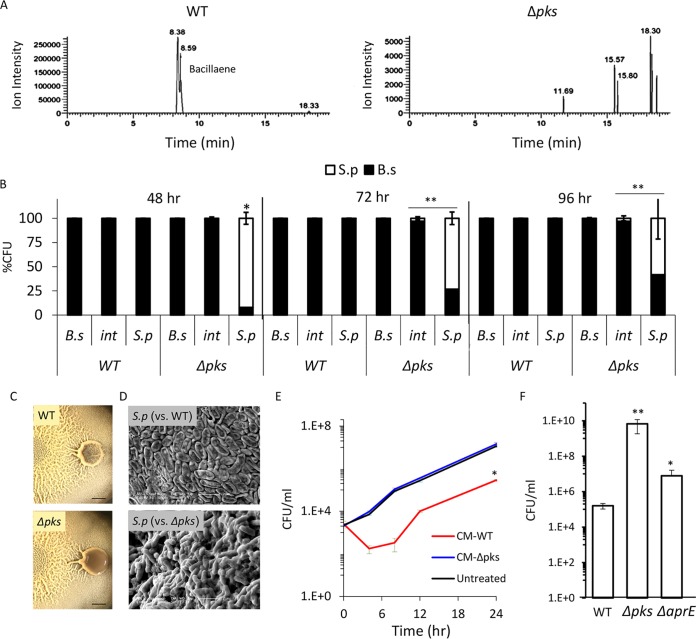
FIG 3.
B. subtilis eliminates S. plymuthica cells in a PKS-dependent manner. (A) Liquid chromatography-mass spectrometry of a wild type (right) and Δpks mutant (left) biofilm colonies extracted with isopropanol. The chromatogram is focused on m/z 581.3585 and related masses. The peaks at retention times of 8.38 min and 8.59 min in the WT extract represent two isomers of bacillaene and are fully absent in the Δpks mutant extract. (B) Relative CFU counts of B. subtilis WT or Δpks mutant and S. plymuthica at each stage of the interaction. The interacting colonies were divided into three areas as follows: B.s, the area of the B. subtilis colony most distant from the interaction area; int, the area of direct interaction; S.p, the area of the S. plymuthica colony most distant from the interaction area. Each section was separately harvested, sonicated, and plated to determine the number of replicative cells of each species. The experiment was repeated at 48 h, 72 h, and 96 h after inoculation. (C) Top-down images of interaction between S. plymuthica and either WT B. subtilis or Δpks mutant after 4 days of incubation. Scale bar = 0.2 cm. (D) SEM images of S. plymuthica from the interaction zone between and either WT B. subtilis or Δpks mutant. Cells were harvested and visualized after 48 h of incubation at 30°C. (E) Growth curves of S. plymuthica in liquid biofilm medium, supplemented with 50% (vol/vol) of B. subtilis WT or Δpks mutant conditioned medium (CM). Experiments were performed at 30°C. (F) The number of CFU represents S. plymuthica replicative cells, at 72 h after coinoculation with WT B. subtilis, Δpks mutant, and ΔaprE mutant strains. *, P < 0.01; **, P < 0.005 based on a two-tailed Student's t test of the entire indicated measurement series. Error bars represent standard deviations. All experiments were performed at least 3 times with at least three technical repeats.