Table 2.
Optimization of reactions
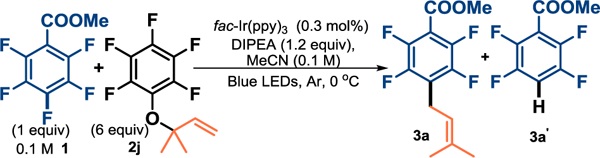 | ||||
|---|---|---|---|---|
| Entry | Modifications | Yield of 3a(%)a | 3a:3a’d | Time (h) |
| 1 | none | 31/62 | 1.6:1 | 5/17 |
| 2 | no cataylst or in dark | 0 | na | 17b |
| 3 | without amine | 0 | na | 17b |
| 4 | −10 °C instead of 0°C | 29/64 | 1.7:1 | 5/25 |
| 5 | DIPEA (1.0 equiv) | 25/57 | 2:1 | 5/23b |
| 6 | DIPEA (1.8 equiv) | 36/64 | 1.9:1 | 5/18 |
| 7 | DIPEA (2.5 equiv) | 38/57 | 1.3:1 | 5/15 |
| 8 | 0.25 mol% catalyst, DIPEA (1.8 equiv) | 31/58 | 1.8:1 | 5/24 |
| 9 | 0.025 mol% catalyst, DIPEA (1.8 equiv) | 6/28 | 1.6:1 | 5/24b |
| 10 | H2O (10 equiv), DIPEA (1.8 equiv) | 57/65 | 1.9:1 | 3/4 |
| 11 | H2O (15 equiv), DIPEA (1.8 equiv) | 59/65 | 1.9:1 | 3/4 |
| 12 | Entry 10 with 0.4 equiv TEMPO | 39/68 | 2.1:1 | 4/10 |
a determined by 19F NMR analysis. Reaction complete unless otherwise noted.
bReaction did not go to completion over extended time, observed 0%, 0%, 83% and 44% conversion of 1 in entries 2, 3, 5 and 9 respectively.
dReported for the final time point.
