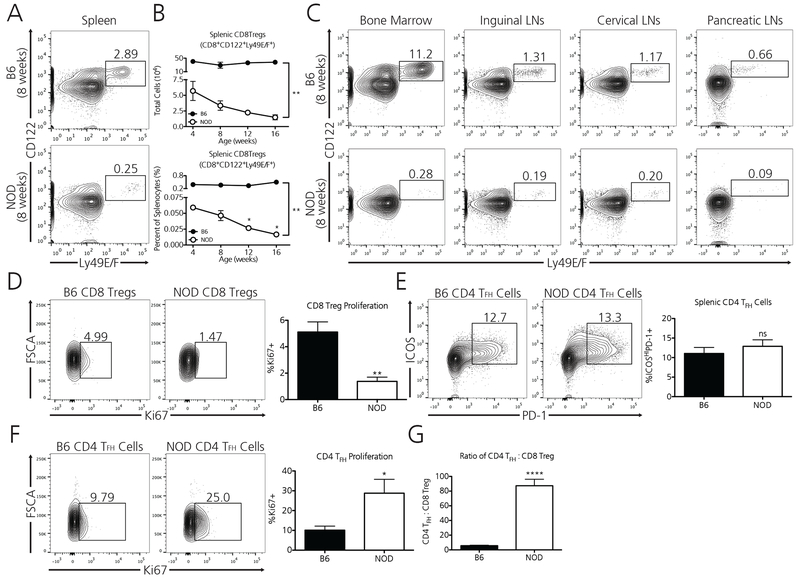
Figure 2. Diabetes-prone NOD mice are profoundly deficient in TFH-targeting Ly49+ CD8 Tregs.
A) Wild-type, 8-week old NOD mice possess 11-fold fewer splenic TFH targeting Ly49+ CD8 Tregs (CD8a+CD122+Ly49E/F+) as compared to non-autoimmune, age-matched B6 mice. B) Whereas healthy B6 mice maintain a relatively robust and stable population of Ly49+ CD8 Tregs as they age, NOD mice progressively lose this protective population as they age toward diabetes. Data represent one experiment which was repeated twice with similar results. N=3 mice per B6 at each age, N=3 mice per NOD at each age. C) The observed Ly49+ CD8 Treg deficiency in NOD mice extends to additional lymphoid compartments including the bone marrow, inguinal lymph nodes, cervical lymph nodes, and pancreatic lymph nodes. Data represent one experiment from pooled immune compartments of B6 (N=3) and NOD (N=3) mice at 8 weeks of age. D) Ly49+ CD8 Tregs in NOD mice divide at a rate three times lower than their B6 counterparts as determined by their Ki67 positivity (Data are from two independent pooled experiments, for a total of N=6 B6 mice and N=6 NOD mice [8 weeks]). Although age-matched NOD mice possess similar percentages of Ly49+ CD8 Treg target TFH cells vs B6 mice (E) (Data pooled from three independent experiments, for a total of N=12 B6 mice and N=11 NOD mice [8 weeks]), TFH cells in NOD mice demonstrate a 2.5-fold higher proliferative rate (F). This discrepancy may account for our observation that CD4 TFH cells outnumber Ly49+ CD8 Tregs in NOD mice approximately 87 to 1 (G) (Data is pooled from two independent experiments, for a total of N=6 B6 mice and N=6 NOD mice [8 weeks]). *p<0.05, **p<0.01, ****p<0.0001, ns = non-significant, by either two-way ANOVA followed by Bonferroni post-test or by student’s t-test.