Figure 6. LMNA in isolated embryonic CMs is sensitive to substrate stiffness and actomyosin stress.
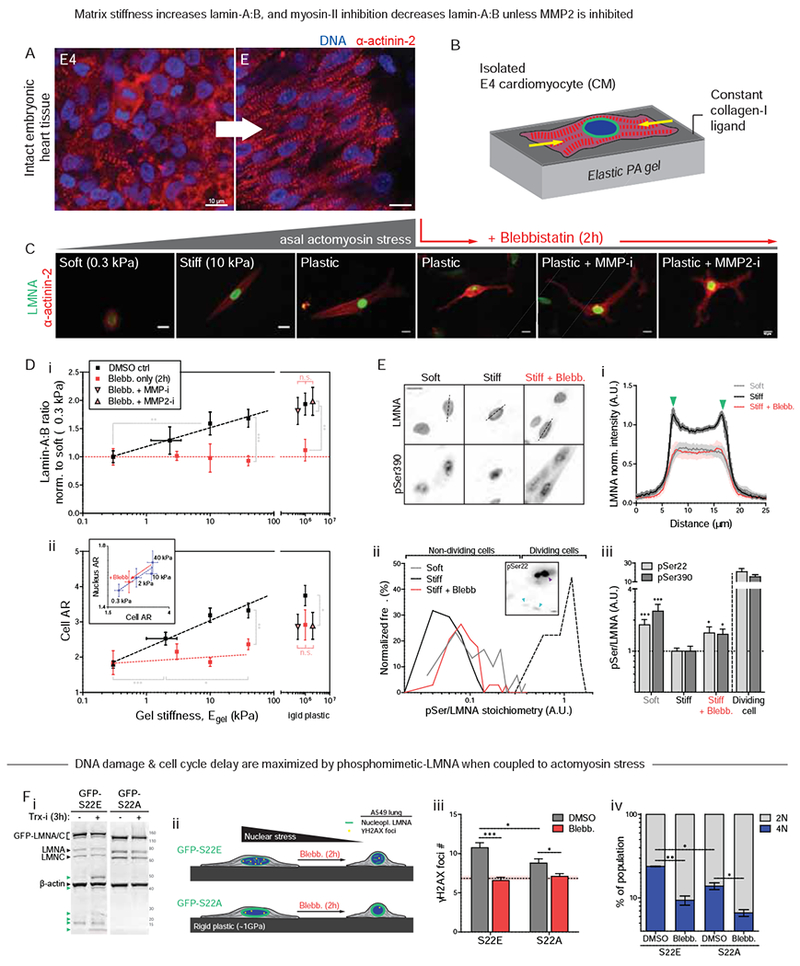
(A) Airyscan images of embryonic heart.
(B) CM cultures on PA gels of controlled stiffness (0.3 −40 kPa) and constant collagen-I ligand.
(C) Well-separated E4 CMs at 24h on gels. As with tissue (A), isolated CMs spread, elongate, and striate more with stiffness. Blebb on rigid plastic causes rounded or dendritic CM, with loss of striation. MMP inhibitors have no effect.
(D) (i) Lamin-A:B increases with gel stiffness except with blebb (n>50 cells per grp). Effects are rescued by MMP-i’s on rigid plastic. (ii) Cell and nuclear AR (inset) increase as CMs polarize on stiffer gels. Blebb reduces cell/nuclear AR, but MMP-i’s show not affect (n>47 cells per cond.; all error bars indicate ±SEM).
(E) (i) LMNA is more nucleoplasmic in CMs on soft gels (0.3 kPa) than stiff (40 kPa). Blebb gives higher nucleoplasmic signal. (ii) Histograms of phosphorylation (‘pSer/LMNA’) for pSer22 and pSer390. Signal for both sites are higher for soft gels and stiff gels + blebb. pSer/LMNA for rare mitotic CMs (yellow) are typically 10~20-fold higher than interphase CMs (cyan arrows). (iii) pSer/LMNA ratios for pSer22 and 390 (n>11 nuclei per cond.).
(F) (i) Phospho-mimetic mutant GFP-S22E (± Trx-i, 3h) probed with anti-LMNA shows low LMNA versus GFP-S22A cells. Stable lines were made within a stable shLMNA line to minimize both endogenous LMNA and overexpression artifacts. Low-MW fragments (green arrows) are not detected in GFP-S22A cells. (ii,iii) Basal DNA damage and (iv) %4N cells are highest in GFP-S22E cells, but blebb minimizes differences (n>93 nuclei per cond.; t-test *p<0.05, **<0.01, ***<0.001; irrelevant blot lanes have been removed and is indicated by a blank space).
