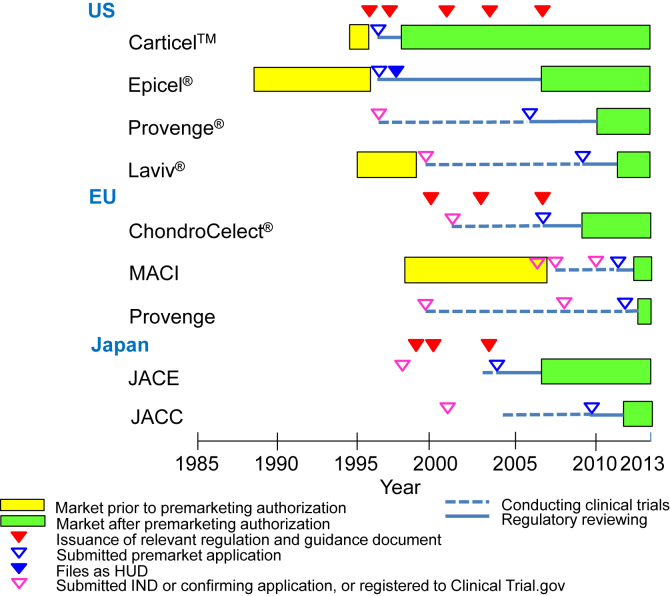
Fig. 1.
Pathway of approved autologous human cells and tissue products. In the US, as the first guidance regarding manipulated autologous structural (MAS) cells was issued on May 28, 1996, three products such as Epicel®, Carticel™ and Laviv® had been on the market. Carticel™ was submitted as the biologics license application (BLA) in 1996, and approved on August 22, 1997 for the accelerated approval of biological products for serious or life-threating illness under 21CFR601.40. Epicel® was filed as the humanitarian device exemption (HDE) application on February 5, 1997, designated as the humanitarian use device (HUD) on November 30, 1998, and approved as the HDE on October 25, 2007. Laviv® was submitted as the BLA on March 6, 2009 after completing clinical trials under the investigational new drugs (IND) on October 12, 1999, and approved as a biologic product on June 21, 2011. Provenge® was submitted to the IND on December 22, 1996 and as the BLA on August 21, 2006 after completing clinical trials, and approved as a biologic product on April 29, 2010. In Japan, after issuing of the notifications with regard to medicinal products using human cells and tissues, JACE was submitted to the new medical device application on October 6, 2004 using clinical trial data collected after confirming preclinical data, and approved as a new medical device on October 29, 2007. JACC was submitted to the new medical device application on August 24, 2009 using clinical trial data collected after confirming preclinical data, and approved as a new medical device on July 27, 2012. In the EU, after issuing the Directive 2001/83/EC (medicinal products directive), the clinical trial of ChondroCelect® was conducted from 2002 to 2006. According to the Regulation (EC) 726/2004 regarding to EU-wide marketing authorization, ChondroCelect® was submitted through the centralized procedure on June 1, 2007, and approved as the ATMP on October 5, 2009. Prior to the introduction of the Regulation (EC) 1394/2007, MACI was available in certain European countries (i.e. Austria, Belgium, Denmark, Germany, Greece, Ireland, Italy, The Netherlands, Norway, Portugal, Spain, and the United Kingdom) and Australia, in accordance with national legislation since 1998. MACI was submitted through the centralized procedure on September 1, 2011, and approved as the ATMP on June 27, 2013. Provenge was submitted through the centralized procedure on December 30, 2011, and approved as the ATMP on September 6, 2013.