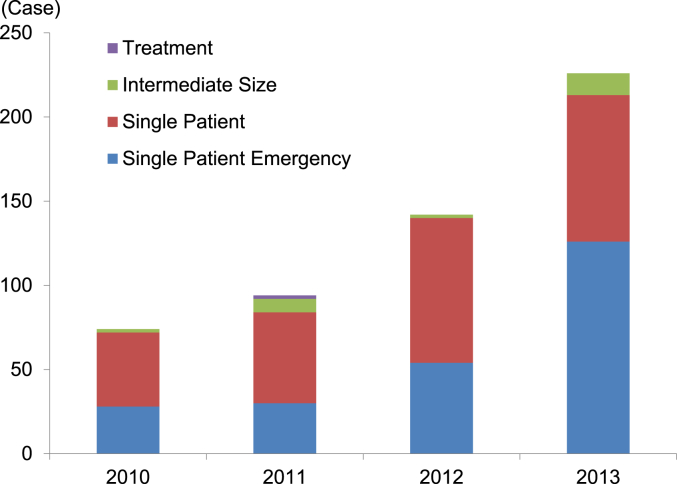
Fig. 5.
The number of FDA expanded access cases for biologics. 2010: reporting year (October 13, 2009–October 12, 2010), 2011: reporting year (October 13, 2010–October 12, 2011), 2012: fiscal year (October 1, 2011–September 30, 2012), 2013: fiscal year (October 1, 2012–September 30, 2013). Source: http://www.fda.gov/biologicsbloodvaccines/ucm413041.htm (accessed January 14, 2015).