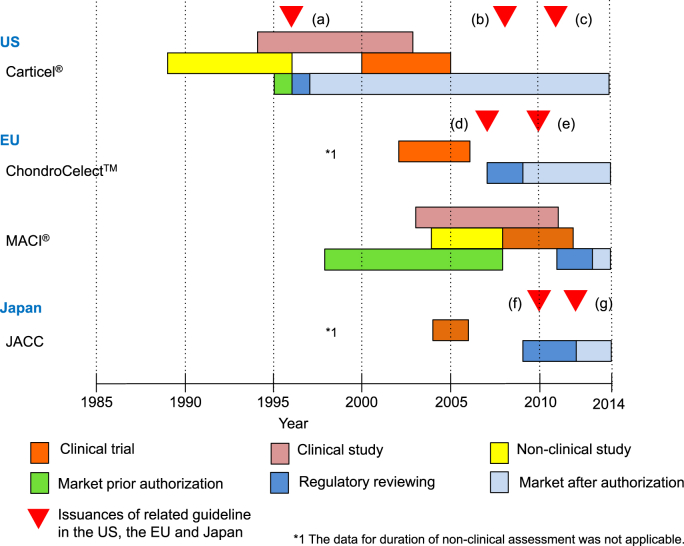
Fig. 1.
Regulatory action regarding approved autologous chondrocyte implantation (ACI) products. In the US, “Guidance on Applications for Products Comprised of Living Autologous Cells Manipulated Ex Vivo and Intended for Structural Repair or Reconstruction; Availability” was released in May 1996 and Carticel® was subsequently approved in August 1997 (a). As the next action, “Guidance for FDA Reviewers and Sponsors Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs)“ was released in April 2008 (b). The latest action was the issuance of the guidelines “Guidance for Industry Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage” in December 2011 (c). In the EU, the “Guideline on human cell-based medicinal products” (EMA/CHMP/410869/2006) was released in January 2007 (d), and the application of ChondroCelect™ was submitted to the EMA in June 2007 and authorized in October 2009. The reflection paper focused on the repair of cartilage lesions of the knee was released in the EMA in April 2010 (e) for the supplement of EMA/CHMP/410869/2006 and was named “Reflection paper on in-vitro cultured chondrocyte containing products for cartilage repair of the knee” (EMA/CAT/CPWP/568181/2009). Latest ACI product was the MACI® authorized in June 2013. In Japan, the application of JACC was submitted to the PMDA in August 2009 and was authorized in July 2012. During the JACC authorization processes the guidance, “The evaluation index for medical device in next generation (Evaluation index about regeneration of articular cartilage)” (f) and “Ensuring the safety and quality of human autologous cell-based or tissue-based pharmaceutical or medical device” (g) by the Ministry of Health Labour and Welfare (MHLW).
References: (a) US Food and Drug Administration. Guidance on Applications for Products Comprised of Living Autologous Cells Manipulated Ex Vivo and Intended for Structural Repair or Reconstruction; Availability. Fed Regist. 1996; 61 FR 26523, May 28, 1996. (b) US Food and Drug Administration. Guidance for FDA Reviewers and Sponsors Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs), April 2008. (c) US Food and Drug Administration. Guidance for Industry Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage. December 2011. (d) The European Medicines Agency. Guideline on human cell-based medicinal product. EMEA/CHMP/410869/2006, January 2007. (e) The European Medicines Agency. Reflection paper on in-vitro cultured chondrocyte containing products for cartilage repair of the knee. EMA/CAT/CPWP/568181/2009, April 2010. (f) The Ministry of Health Labour and Welfare. The evaluation index for medical device in next generation (Evaluation index about regeneration of articular cartilage). Yakushokuki-hatsu 1215 No.1, December 2010. (g) The Ministry of Health Labour and Welfare. Ensuring the safety and quality of human autologous cell-based or tissue-based pharmaceutical or medical device. Yakushokuki-hatsu 0907 No.2, September 2012.