Abstract
Introduction
Bone marrow mesenchymal stem cells (BMMSCs) ameliorate tissue damage after ischemic injury. Erythropoietin (Epo) has pleiotropic effects in addition to hematopoietic activity. The aim of this study was to investigate whether Epo enhanced cell survival and angiogenic effect of BMMSC implantation in rat limb ischemia model.
Methods and results
MSCs were isolated from BM in GFP-transgenic rats. In a culture study, Epo promoted BMMSC proliferation in normoxia and enhanced cell survival under the culture condition mimicking ischemia (1% oxygen and nutrient deprivation). BMMSCs with and without 48 h of pretreatment by Epo (80 IU/ml) were locally administered to rat hindlimb ischemia models in vivo. At 3 days after implantation, BMMSC engraftment in the perivascular area of the injured muscle was significantly higher in the cells preconditioned with Epo than in the cells without preconditioning. Stromal derived factor-1α and fibroblast growth factor-2 expressions were detected in the engrafted BMMSCs. At 14 days after implantation, the Epo-preconditioned BMMSCs significantly promoted blood perfusion and capillary growth compared to the controls in laser Doppler and histological studies. In addition to promoting neovascularization, the Epo-preconditioned BMMSCs significantly inhibited macrophage infiltration in the perivascular area.
Conclusion
Epo elicited pro-survival potential in the BMMSCs. Pharmacological cell modification with Epo before implantation may become a feasible and promising strategy for improving current therapeutic angiogenesis with BMMSCs.
Keywords: Mesenchymal stem cells, Erythropoieitn, Angiogenesis, Cell engraftment
Highlights
-
•
Erythropoietin rescued the BMMSCs against the culture condition mimicking ischemia.
-
•
Erythropoietin promoted cellular engraftment of the BMMSCs in rat ischemic limbs.
-
•
Preconditioning with erythropoietin enhanced angiogenic effects of the BMMSC implantation.
1. Introduction
Therapeutic angiogenesis with bone marrow mononuclear cells has shown promise as a less invasive intervention for no-option patients with critical limb ischemia [1]. Optimism is guarded, however, as the cell therapy has failed to confer sufficient effects on limb salvage in patients with atherosclerotic critical limb ischemia [2]. Among the bone marrow cells used in clinical cell therapy, our group previously demonstrated the important contributions of stem/progenitor cells in ameliorating limb ischemia [3].
Bone marrow mesenchymal stem cells (BMMSCs) are multipotent and capable of secreting pro-angiogenic and cytoprotective factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)-2 [4]. Accumulating evidence from animal studies has shown that BMMSC implantation promotes angiogenesis and tissue repair in injured organs [5], [6], [7], [8], [9]. Previous studies by our group have also demonstrated the therapeutic efficacy of BMMSCs in animal models of cardiovascular disease [10], [11], [12], [13].
Two important challenges, however, still impede the effectiveness of BMMSC implantation. The first is the marginal or incomplete grafting of BMMSCs additionally injected into ischemic tissues. The second is the scarcity of clinically relevant methods to further enhance the tissue-repairing properties of BMMSCs. These challenges may be surmountable by modifying the paracrine effects of the implanted cells in order to protect and preserve the injured tissues [14]. Many investigators have attempted to modify implanted BMMSCs using gene engineering techniques and/or particular culture conditions [15], [16], [17]. Few, however, have reported on the implantation of BMMSCs modified by pharmacological preconditioning for clinical application [18], [19].
Erythropoietin (Epo) was first characterized as a hematopoietic factor that stimulates the differentiation of hematopoietic progenitor cells into erythroid cells [20], [21], [22], [23]. Epo is widely administered in clinical settings to promote the production of red blood cells in patients with anemia caused by chronic kidney disease. Besides stimulating erythropoiesis, Epo has elicited pleiotropic effects such as anti-apoptosis in various types of cells [20], [23]. We therefore speculated that Epo would also be effective in protecting and activating BMMSCs.
In the present study we investigated whether pharmacological cell modification by priming with Epo promoted the paracrine effects of BMMSCs or enhanced the effectiveness of BMMSC implantation.
2. Materials and methods
2.1. Isolation and culture of GFP-transgenic BMMSCs
We isolated and cultured the BMMSCs of adult transgenic Sprague–Dawley rats ubiquitously expressing enhanced green fluorescent protein (GFP), as previously described [13], [24]. Briefly, mononuclear cells were purified from GFP-rat bone marrow by density centrifugation and re-suspended in a complete culture medium (CCM) consisting of alpha-MEM (Life Technologies, CA, US) with 20% fetal bovine serum (Atlanta Biologicals, GA, US), 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM l-glutamine. The cells were cultured in 20 ml CCM of a 150 cm2 culture dish and incubated in a humidified incubator with 95% air and 5% CO2 at 37 °C. After 24 h, the non-adherent cells were removed and the primary adherent cells were cultured and propagated. Passages 3 to 7 were used for the experiments.
2.2. Proliferation and survival assays
Recombinant erythropoietin was kindly provided by Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan). For a proliferation assay, BMMSCs were plated separately at a density of 5,000 cells/cm2 in 20 ml of CCM in a 150 cm2 culture dish. The cells were cultured in a normoxic humidified incubator (ASTEC, SCA-165DS) with 95% air and 5% CO2 at 37 °C and then treated with various concentrations of Epo (0, 40, 80 IU/ml) (n = 3). After 5 days, the cells were deattached by trypsin and counted. The cell number ratio was defined as the ratio of cell counts at baseline and 5 days after the treatment.
For a survival assay, BMMSCs were plated separately at a density of 5,000 cells/cm2 in 20 ml of CCM in a 150 cm2 culture dish. After reaching 50% confluence in a normoxic condition, the cells were treated with serum-free medium with or without 80 IU/ml Epo and cultured in a hypoxic condition with 1% O2, 5% CO2, and 94% N2 for 48 h (n = 3). After 48 h, the cells were deattached by trypsin and counted. The cell number ratio was defined as the ratio of cell counts before and 48 h after the treatment under the condition with hypoxia and nutrient depletion.
2.3. Rat hindlimb ischemic model and cell implantation
This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The Animal Care and Use Committee of Showa University approved the experimental protocol.
We created hindlimb ischemia models of 8-week-old male rats as previously described [13], [25]. In brief, the right femoral arteries of the animals were ligated under anesthesia. The distal portion of the saphenous artery and all of the side branches were ligated, along with the vein. The left hindlimb was kept intact and used as a non-ischemic limb. After the operation, BMMSCs preconditioned with or without EPO were injected into the ischemic adductor muscles at four sites (Epo-preconditioned group, 5 × 105 cells, n = 15; Control, 5 × 105 cells, n = 14). The preconditioned BMMSCs were treated with Epo at a concentration of 80 IU/ml for 48 h before the injection. Epo (5000 IU/kg) was simultaneously injected into the ischemic limbs during the cell implantation in both groups.
Blood perfusion was assessed by laser Doppler perfusion imaging (LDPI) (Omega Zone, Tokyo, Japan) before and at 14 days after the implantation (n = 11 in each group). Hair in lower limbs was carefully and completely removed before scanning. The blood flow distribution of the limb was then mapped out as a color-coded image directly proportional to the blood flow perfusion. Area of the lower limbs (femur and the below knee part) was traced, and the traced images were analyzed to quantify blood flow. The LDPI index was used to calculate the blood perfusion ratio of the ischemic and non-ischemic hindlimbs [13], [25]. Tissue samples were obtained from rat ischemic adductor muscles at 3 and 14 days after surgery for immunohistochemistry.
2.4. Immunohistochemistry
Immunohistochemical staining of the hindlimb sections was performed as previously described [13], [25]. Tissue sections were incubated with primary antibodies overnight at 4 °C, washed in PBS thrice, and incubated with peroxidase-labeled anti-rabbit or anti-mouse antibody (Histofine Simplestain Max PO; Nichirei, Tokyo, Japan). The binding antibody was finally visualized by 3,3′-diaminobenzidine staining followed by counter-staining with hematoxylin. The primary antibodies were raised against CD31 at a 1:100 dilution (DAKO, CA, USA), GFP at a 1:1000 dilution (Life Technologies), and RM-4 at a 1:1000 dilution (Trans Genic Inc., Hyogo, Japan).
To assess the capillary density, three fields from each tissue section (n = 6) were randomly selected and the number of CD31-positive cells was counted in each field. To avoid over- or underestimating the capillaries as a consequence of myocyte atrophy or interstitial edema, the number of capillaries adjusted per muscle fiber was used to compare differences in the capillary density [13], [25].
To assess engrafted GFP-positive BMMSCs in injured muscles at 3 days after transplantation, three fields (200 × 250 μm2/field) from each section (n = 3 to 4) were randomly selected and the number of GFP-positive cells was counted in each field.
The anti-rat macrophage monoclonal antibody, RM-4, is used for identifying macrophages. RM-4 recognizes a membrane protein of endolysosomes in macrophages [26]. To assess macrophage infiltration into ischemic limb sections on postimplantation day 14, three fields (200 × 250 μm2/field) from each tissue section (n = 7 to 8) were randomly selected and the number of RM-4-positive cells in the perivascular area was counted in each field.
2.5. Immunofluorescence
The BMMSCs were plated at a density of 5000 cells/cm2 in 2 ml of CCM in a Chamber Slide® (Lab-Tak, CO, USA). After 48 h of culture in a normoxic humidified incubator, the cells were sequentially washed with PBS and treated with 0.2%Triton-100 for 15 min in three repeated cycles. The slides were blocked in 2% goat serum at room temperature for 1 h, incubated overnight with Epo receptor (Epo-R) (1:1000, Novus Biologicals, CO, USA) and raised against GFP (1:1000) diluted in PBS. After rinsing with PBS, the slides were treated with Alexa Fluor® 488 (1:200) and Alexa Fluor® 594 (1:400) (Invitrogen, CA, USA) for 1 h at room temperature. After 3 washes with PBS, the dishes were mounted, cover slipped, and photographed under a florescent microscope DP73 (Olympus, Tokyo, Japan).
The immunofluorescence of the histological sections was performed as previously described [10]. The primary antibodies were raised against GFP at a 1:1000 dilution, stromal derived factor (SDF)-1α at a 1:2000 dilution (Santa Cruz Biotechnology, CA, USA), FGF-2 at a 1:1000 dilution (Santa Cruz Biotechnology), CD34 at a 1:400 dilution (abcam, MA, USA), and KDR/Flk-1 at a 1:400 dilution (abcam, MA, USA). The secondary antibodies used were raised against Alexa Fluor® 488 (1:200) and Alexa Fluor® 594 (1:400) (Invitrogen, CA, USA).
2.6. Statistical analysis
All data were expressed as means ± SD. Comparisons between two groups were performed using unpaired student's t -tests. P < 0.05 was considered significant.
3. Results
3.1. The Epo/Epo-R system promoted proliferation and survival of cultured BMMSCs
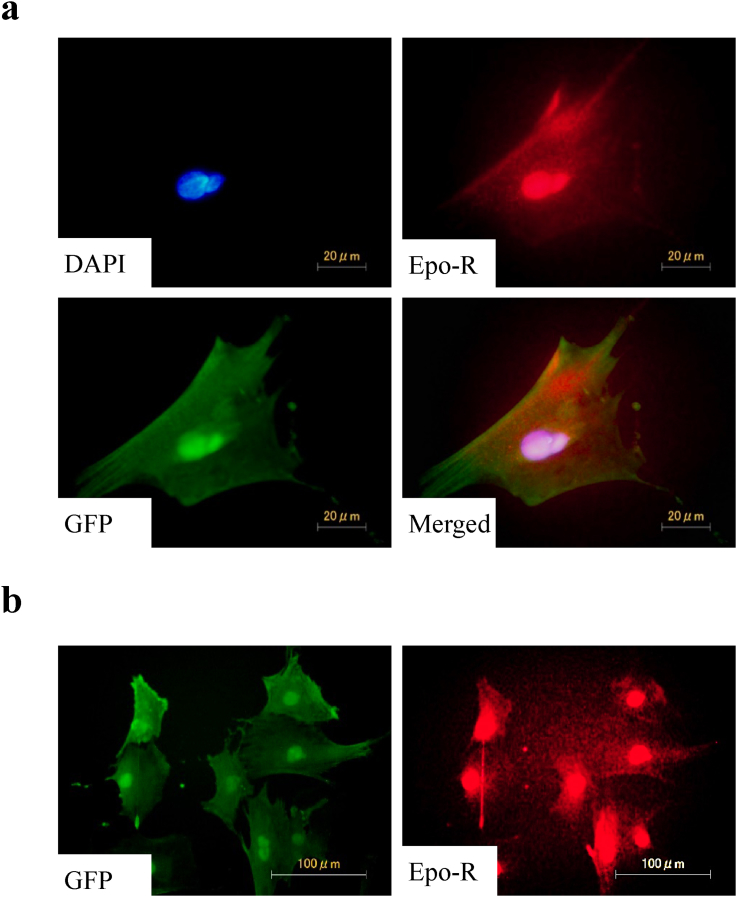
BMMSCs from the GFP transgenic rats were immunostained using antibodies against GFP and Epo-R. The immunostaining revealed the expression of Epo-R in GFP-positive BMMSCs (Fig. 1a). In our observation, Epo-R ubiquitously expressed in the BMMSCs (Fig. 1b).
Fig. 1.
Epo-R expression in BMMSCs from GFP rats. Epo-R (red) expression in GFP (green)-positive BMMSCs. (a) Epo-R expression in single cell. (b) Epo-R was ubiquitously expressed in BMMSCs. Nuclei were stained with DAPI (blue). BMMSCs, bone marrow mesenchymal stem cells; DAPI, 4′,6-Diamidino-2-phenylindole; Epo-R, erythropoietin receptor; GFP, green fluorescent protein.
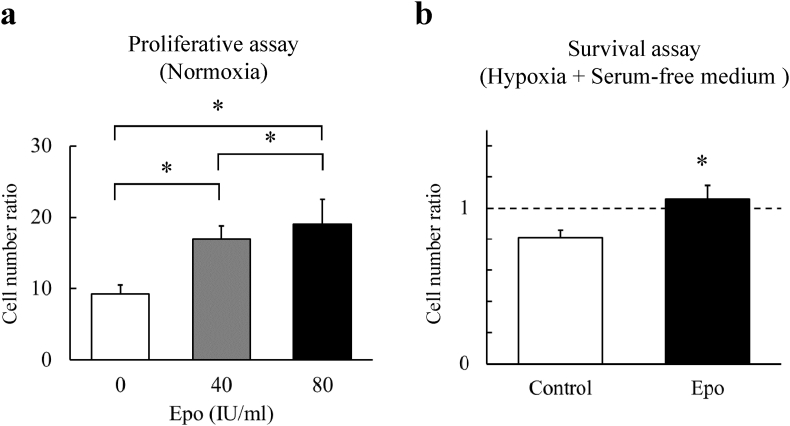
We examined whether the Epo influenced propagation in the BMMSCs under a standard culture condition. The BMMSCs were cultured with Epo at concentrations of 0 (control), 40, and 80 IU/ml in the CCM for 5 days. By the end of the culture on day 5, Epo had significantly promoted the proliferation of the BMMSCs compared with the controls in a dose-dependent fashion (p < 0.05 for each dose) (Fig. 2a).
Fig. 2.
Survival effect of Epo in BMMSCs. (a) Epo significantly increased the number of BMMSCs under standard culture condition in dose dependent fashion. The cell number ratio was defined as the ratio of cell counts at baseline and 5 days after the treatment. N = 6 in each group *, p < 0.05. (b) Epo protected the BMMSCs against the condition with hypoxia (1%O2) and serum deprivation. The cell number ratio was defined as the ratio of cell counts before and 48 h after the treatment under the condition with hypoxia and nutrient depletion. N = 6 in each group *, p < 0.05.
Because cells transplanted to the injured tissues may encounter ischemic environments, we next examined whether Epo could protect BMMSCs under a condition mimicking ischemia (hypoxia (1% oxygen) and nutrient deprivation) for 48 h ex vivo. The rate of untreated BMMSC survival was 81 ± 4% compared to the baseline, whereas that of BMMSCs treated with 80 IU/ml Epo was 105 ± 4% (p < 0.05) (Fig. 2b).
This result demonstrated the proliferative and protective effects of the Epo/Epo-R system on non-hematopoietic stem cells. The Epo treatment appeared to enhance MSC engraftment in ischemic tissues.
3.2. Priming with Epo enhanced the cell survival and angiogenic effect of BMMSC implantation
The results of our in vitro study encouraged us to attempt an investigation into the in vivo effects of BMMSCs preconditioned with Epo. Specifically, we investigated whether priming with Epo ameliorated the cell survival and angiogenic effect of BMMSCs in a rat model of limb ischemia, compared with BMMSCs implanted without pretreatment.
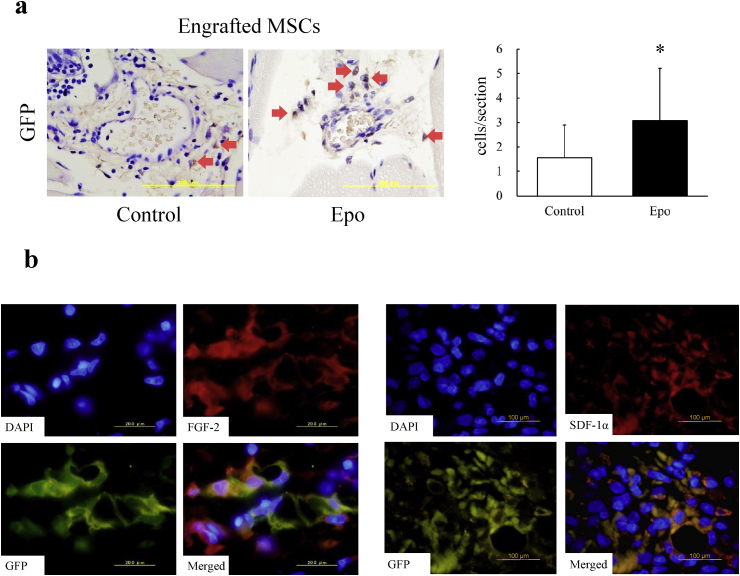
Our previous study demonstrated that BMMSC implantation promoted neovascularization even in the early phase (day 3) after the treatment [13]. However, the engrafted cells was not detected after 7 days. Thus, we evaluated the MSC engraftment at day 3 after the implantation. BMMSCs positive for GFP were detected in the perivascular area of the muscle tissue 3 days after the cell implantation (Fig. 3a). Counting the number of GFP-positive cells in muscle sections was performed with the use of 3,3′-diaminobenzidine staining followed by counter-staining with hematoxylin. The engrafted BMMSCs preconditioned with Epo significantly outnumbered the control BMMSCs (p < 0.05). Immunofluorescence staining was performed to detect angiogenic factors in the engrafted BMMSCs. GFP-and-FGF-2 and GFP-and-SDF-1α double-positive cells were both found in ischemic muscles after the implantation (Fig. 3b).
Fig. 3.
BMMSC engraftment in rat ischemic muscles at 3 days after implantation. (a) Left, immunohistochemical images of engrafted BMMSCs stained with GFP antibody. Right, priming with Epo significantly increased the number of engrafted BMMSCs in ischemic hindlimb muscles. N = 3 to 4. *, p < 0.05. (b) Secretion of FGF-2 and SDF-1α from engrafted BMMSCs. Left, immunofluorescence of FGF-2 (red) and GFP (green). FGF-2 and GFP double-positive cells were found in ischemic muscles on day 3 after implantation. Nuclei was stained with DAPI (blue). Bar = 20 μm. Right, immunofluorescence of SDF-1α (red) and GFP (green). SDF-1α and GFP double-positive cells were found in ischemic muscles on day 3 after implantation. Nuclei were stained with DAPI (blue). Bar = 100 μm.
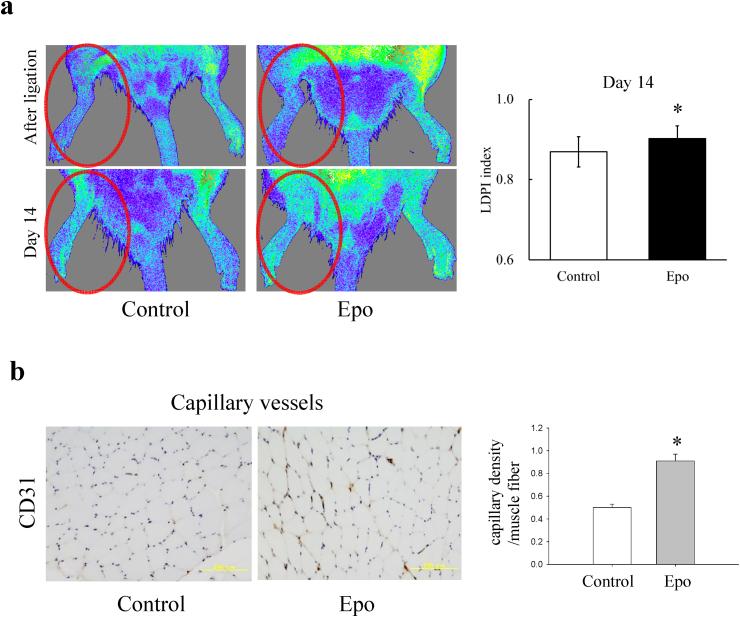
Blood perfusion recovery of the ischemic hindlimb was evaluated by LDPI before and on day 14 after the implantation. Blood perfusion before the implantation (immediately after the ligation) was decreased in the both groups (Fig. 4a), and the LDPI index was not significantly different between the two groups (0.55 ± 0.06 vs 0.58 ± 0.05). Hindlimb ischemia on day 14 after the implantation was markedly improved in the Epo-preconditioned group compared with that in the control group (Fig. 4a). A quantitative analysis of the LDPI findings revealed significantly higher perfusion recovery in the Epo-preconditioned MSCs than in the controls (p < 0.05). Apart from the LDPI, a histological study was performed to assess the capillary density in the muscle on day 14 after the treatment (Fig. 4b). The capillary/muscle fiber ratio in ischemic muscle was significantly higher in the Epo-preconditioned group than in the control group (p < 0.05).
Fig. 4.
Blood flow recovery and capillary density after BMMSC implantation. (a) Left, representative images of laser Doppler blood flow (LDPI) in the control group and Epo-preconditioned group. Right, the LDPI index was used to calculate the blood perfusion ratio of the ischemic and non-ischemic hindlimbs. Blood perfusion signals of the ischemic limb were significantly higher in the Epo-pretreated BMMSC implantation group than in the control on day 14. N = 11 in each group. *, p < 0.05. (b) Left, immunohistochemical staining for CD31 in muscle sections on day 14 after the ligation. Bar = 200 μm. Right, the capillary/muscle fiber ratio was significantly higher in the Epo-pretreated BMMSC implantation group than in the controls. N = 6 in each group. *, p < 0.05.
An earlier study by our group demonstrated that BMMSC implantation promoted angiogenesis in rat ischemic limbs without persistent cell engraftment [13]. The preconditioning with Epo in the present study was thus found to further promote the beneficial effects of the BMMSC implantation.
3.3. Efficacy of Epo-preconditioned BMMSCs on infiltration of bone marrow derived cells into ischemic limbs
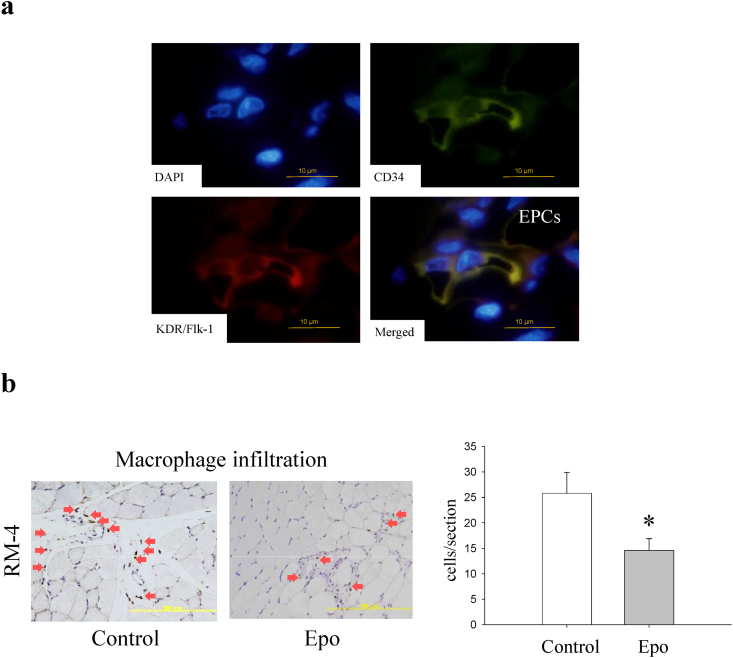
The SDF-1α chemokine is known to induce the mobilization of endothelial progenitor cells (EPCs) [27]. EPCs are generally characterized by the cell surface expression of CD34 and KDR/Flk-1 [27]. A number of CD34-and-KDR/Flk-1 double-positive EPCs were detected in the muscles injected with the Epo-preconditioned BMMSCs (Fig. 5a), while no EPCs appeared in the controls. This finding suggested that the increased BMMSC engraftment by Epo priming induced to mobilize EPCs via increased production of SDF-1α in the muscle tissue as compared with the BMMSCs without preconditioning.
Fig. 5.
Mobilization of bone-marrow derived cells into ischemic muscles. (a) Migrated EPCs in rat ischemic muscles treated with Epo-preconditioned BMMSCs. Immunofluorescence of KDR/Flk-1 (red) and CD34 (green), EPC markers. EPCs were found in ischemic muscles treated with the Epo-preconditioned BMMSCs on day 3 after implantation. Nuclei were stained with DAPI (blue). Bar = 10 μm. EPC, endothelial progenitor cell. (b) Macrophage infiltration in ischemic muscles after BMMSC implantation. Left, immunohistochemical images of macrophages stained with the RM-4 antibody on day 14 after implantation. Red arrows indicate RM-4 positive cells. Bar = 200 μm. Right, macrophage counts were significantly lower in the Epo-preconditioned group (n = 7) than in the control group (n = 8). *, p < 0.05.
Factors secreted from BMMSCs preconditioned with Epo may or may not attract inflammatory cells as efficiently as EPCs. To investigate further, we evaluated macrophage infiltration into the muscles 14 days after the cell implantation (Fig. 5b). Macrophage counts were significantly lower in the Epo-preconditioned group than in the control group (p < 0.05). This result suggested that the Epo-preconditioning ameliorated the anti-inflammatory properties of the BMMSCs.
4. Discussion
A few reports have demonstrated tissue-repair effects in tissues injected with stem cells and Epo in combination [28], [29]. None of them, however, elucidated the precise mechanisms of the effects. Now, in our present culture study, we find that the Epo/Epo-R system promotes BMMSC proliferation under regular conditions and BMMSC survival under conditions with hypoxia and serum depletion. Based on the results, we attempted a preconditioning strategy in addition to co-administering the cells and reagent, in rat ischemic limbs. The BMMSCs preconditioned with Epo induced more neovascularization than the unconditioned BMMSCs by enhancing cell engraftment.
Our previous clinical study showed the efficacy of a single dose of Epo in treating patients with acute myocardial infarction [30]. We speculate that the mechanisms underlying the benefits may be responsible for the anti-apoptotic and tissue-protective effects of Epo on the myocardium. Animal studies support this hypothesis. Epo administration has exhibited several actions independent of its hematopoietic activity, including the repression of myocardial cell death in a rabbit myocardial infarction model [31], an acute cardioprotective effect in rat ischemia–reperfusion injuries [32], and the prevention of cardiac dysfunction in doxorubicin-induced cardiomyopathy in mice [33].
Poor graft success is a common problem after implantation of cultured cells into injured tissues and occurs with implants of adult stem cells, embryonic stem cells, and cell derivatives [14], [34], [35]. Recent efforts to improve graft success have utilized genetic manipulation to over-express pro-survival factors such as Akt in implanted cells or co-administer cells with accessory scaffolds to support the graft [36], [37]. Epo treatment in the present study rescued BMMSCs from cell death under culture conditions with hypoxia and serum depletion. Earlier we reported improved limb perfusion even in the absence of persistent BMMSC engraftment in rat ischemic limbs. We now find that short-term pharmacological (non-genetic) modification with Epo appears to induce more neovascularization, partly via its effect in promoting the cell engraftment.
The secretion of paracrine factors that alter the tissue microenvironment may play a more prominent role in BMMSC-induced tissue and organ repair than cell transdifferentiation [38]. BMMSCs express a number of proangiogenic factors, as well as proteins that modulate endothelial cell migration [10], [11]. In our experiments comparing cultured human BMMSCs with hematopoietic stem cells, the former expressed higher mRNA levels for proangiogenic factors such as VEGF [10]. VEGF plays a demonstrable role as a crucial mediator of MSC-mediated effects in the injured rat myocardium [39].
The BMMSCs engrafted in the ischemic muscle produced SDF-1α and FGF-2. The factors secreted from BMMSCs play critical roles in angiogenesis and vasculogenesis in ischemic tissues, as well as VEGF. FGF-2 enhances functional neovascularization in ischemic tissues via not only angiogenesis and arteriogenesis, but also synergistic effects with VEGF [40]. SDF-1α stimulates endothelial cell migration, EPC mobilization, and the associated enhancement of angiogenesis in damaged vessels and hearts [31], [41]. Increased number of engrafted BMMSCs in the Epo preconditioning group may produce higher levels of the angiogenic cytokines as compared with the unconditioned BMMSC group. Epo priming also appeared to exert anti-inflammatory potential in the BMMSCs.
In conclusion, short-term preconditioning with Epo elicited pro-survival potential in BMMSCs. Cell modification with pharmacological priming before the implantation may become a feasible and promising strategy for improving therapeutic angiogenesis with cells.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science [to Y Iso (22790732) and H Suzuki (24591072)].
Conflict of interest
None declared.
Acknowledgment
We thank Izumi Yamada and Sayaka Usui for their excellent technical assistance.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Tateishi-Yuyama E., Matsubara H., Murohara T., Ikeda U., Shintani S., Masaki H. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 2.Matoba S., Tatsumi T., Murohara T., Imaizumi T., Katsuda Y., Ito M. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Iso Y., Soda T., Sato T., Sato R., Kusuyama T., Omori Y. Impact of implanted bone marrow progenitor cell composition on limb salvage after cell implantation in patients with critical limb ischemia. Atherosclerosis. 2010;209:167–172. doi: 10.1016/j.atherosclerosis.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Bronckaers A., Hilkens P., Martens W., Gervois P., Ratajczak J., Struys T. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143:181–196. doi: 10.1016/j.pharmthera.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Kinnaird T., Burnett E.S., Shou M., Lee C.W., Barr S., Fuchs S. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 6.Williams A.R., Hare J.M. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morigi M., Imberti B., Zoja C., Corna D., Tomasoni S., Abbate M. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz L.a., Gambelli F., McBride C., Gaupp D., Baddoo M., Kaminski N. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurozumi K., Nakamura K., Tamiya T., Kawano Y., Ishii K., Kobune M. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Iso Y., Spees J.L., Serrano C., Bakondi B., Pochampally R., Song Y.-H. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato T., Iso Y., Uyama T., Kawachi K., Wakabayashi K., Omori Y. Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab Invest. 2011;91:553–564. doi: 10.1038/labinvest.2010.202. [DOI] [PubMed] [Google Scholar]
- 12.Iso Y., Yamaya S., Sato T., Poole C.N., Isoyama K., Mimura M. Distinct mobilization of circulating CD271+ mesenchymal progenitors from hematopoietic progenitors during aging and after myocardial infarction. Stem Cells Transl Med. 2012;1:462–468. doi: 10.5966/sctm.2011-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usui S., Iso Y., Sasai M. Mesenchymal stem cells from bone marrow enhance neovascularization and stromal cell proliferation in rat ischemic limb in the early phase after implantation. Showa Univ J Med Sci. 2014;26:121–129. [Google Scholar]
- 14.Shimada I.S., Spees J.L. Stem and progenitor cells for neurological repair: minor issues, major hurdles, and exciting opportunities for paracrine-based therapeutics. J Cell Biochem. 2011;112:374–380. doi: 10.1002/jcb.22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzau V.J., Gnecchi M., Pachori A.S. Enhancing stem cell therapy through genetic modification. J Am Coll Cardiol. 2005;46:1351–1353. doi: 10.1016/j.jacc.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Gnecchi M., He H., Liang O.D., Melo L.G., Morello F., Mu H. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 17.Wei L., Fraser J.L., Lu Z.-Y., Hu X., Yu S.P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Numasawa Y., Kimura T., Miyoshi S., Nishiyama N., Hida N., Tsuji H. Treatment of human mesenchymal stem cells with angiotensin receptor blocker improved efficiency of cardiomyogenic transdifferentiation and improved cardiac function via angiogenesis. Stem Cells. 2011;29:1405–1414. doi: 10.1002/stem.691. [DOI] [PubMed] [Google Scholar]
- 19.Shinmura D., Togashi I., Miyoshi S., Nishiyama N., Hida N., Tsuji H. Pretreatment of human mesenchymal stem cells with pioglitazone improved efficiency of cardiomyogenic transdifferentiation and cardiac function. Stem Cells. 2011;29:357–366. doi: 10.1002/stem.574. [DOI] [PubMed] [Google Scholar]
- 20.Broxmeyer H.E. 2013. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration; pp. 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelkmann W. Erythropoietin: back to basics. Blood. 2010;115:4151–4152. doi: 10.1182/blood-2010-03-271395. [DOI] [PubMed] [Google Scholar]
- 22.Jelkmann W., Elliott S. Erythropoietin and the vascular wall: the controversy continues. Nutr Metab Cardiovasc Dis. 2012:1–7. doi: 10.1016/j.numecd.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Nairz M., Sonnweber T., Schroll A., Theurl I., Weiss G. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 2012;14:238–246. doi: 10.1016/j.micinf.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendirci M., Trost L., Bakondi B., Whitney M.J., Hellstrom W.J.G., Spees J.L. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010;184:1560–1566. doi: 10.1016/j.juro.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usui S., Iso Y., Sasai M., Mizukami T., Mori H., Watanabe T. Kisspeptin-10 induces endothelial cellular senescence and impaired endothelial cell growth. Clin Sci. 2014;127:47–55. doi: 10.1042/CS20130505. [DOI] [PubMed] [Google Scholar]
- 26.Iyonaga K., Takeya M., Yamamoto T., Ando M., Takahashi K. A novel monoclonal antibody, RM-4, specifically recognizes rat macrophages and dendritic cells in formalin-fixed, paraffin-embedded tissues. Histochem J. 1997;29:105–116. doi: 10.1023/a:1026477104227. [DOI] [PubMed] [Google Scholar]
- 27.Hristov M., Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res. 2008;58:148–151. doi: 10.1016/j.phrs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Esneault E., Pacary E., Eddi D., Freret T., Tixier E., Toutain J. Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2008;28:1552–1563. doi: 10.1038/jcbfm.2008.40. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D., Zhang F., Zhang Y., Gao X., Li C., Ma W. Erythropoietin enhances the angiogenic potency of autologous bone marrow stromal cells in a rat model of myocardial infarction. Cardiology. 2007;108:228–236. doi: 10.1159/000096803. [DOI] [PubMed] [Google Scholar]
- 30.Ozawa T., Toba K., Suzuki H., Kato K., Iso Y., Akutsu Y. Single-Dose Intravenous administration of recombinant human erythropoietin is a promising treatment for patients with acute myocardial infarction. Circ J. 2010;74:1415–1423. doi: 10.1253/circj.cj-10-0109. [DOI] [PubMed] [Google Scholar]
- 31.Parsa C.J., Matsumoto A., Kim J., Riel R.U., Pascal L.S., Walton G.B. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright G.L., Hanlon P., Amin K., Steenbergen C., Murphy E., Arcasoy M.O. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J. 2004;18:1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- 33.Li L., Takemura G., Li Y., Miyata S., Esaki M., Okada H. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–543. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- 34.Mohsin S., Siddiqi S., Collins B., Sussman M.A. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011;109:1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robey T.E., Saiget M.K., Reinecke H., Murry C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segers V.F.M., Lee R.T. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 37.Mangi A.a., Noiseux N., Kong D., He H., Rezvani M., Ingwall J.S. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 38.Phinney D.G., Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 39.Markel T.a., Wang Y., Herrmann J.L., Crisostomo P.R., Wang M., Novotny N.M. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol. 2008;295:H2308–H2314. doi: 10.1152/ajpheart.00565.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kondoh K., Koyama H., Miyata T., Takato T., Hamada H., Shigematsu H. Conduction performance of collateral vessels induced by vascular endothelial growth factor or basic fibroblast growth factor. Cardiovasc Res. 2004;61:132–142. doi: 10.1016/j.cardiores.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi J.I., Kusano K.F., Masuo O., Kawamoto A., Silver M., Murasawa S. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]