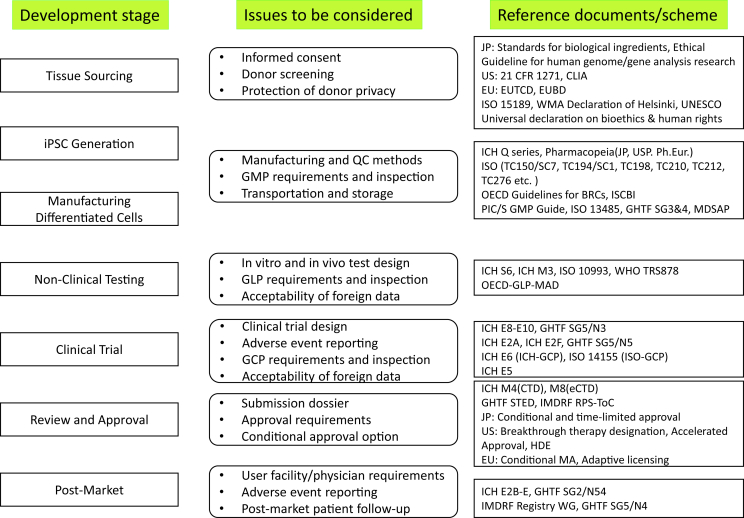
Fig. 4.
Overview of development stage, issues and major reference documents/schemes related to iPSC-based regenerative medicine products. CLIA, Clinical Laboratory Improvement Amendments; EUTCD, European Union Tissues and Cells Directives (2004/23); EUBD, European Union Blood Directive (2002/98); WMA, World Medical Association; UNESCO, United Nations Educational, Scientific and Cultural Organization; ICH, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; JP, Japanese Pharmacopoeia; USP, United States Pharmacopeia; Ph.Eur, European Pharmacopoeia; ISO, International Organisation for Standardization; TC, Technical Committee; SC, Subcommittee OECD, Organisation for Economic Co-operation and Development; GL, Guideline; BRC, Biological Resource Centres; ISCBI, International Stem Cell Banking Initiative; PIC/S, Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme; GMP, Good Manufacturing Practices; GHTF, Global Harmonization Task Force; SG, Study Group; MDSAP, Medical Device Single Audit Program; WHO, World Health Organization; TRS, Technical Report Series; GLP, Good Laboratory Practice; MAD, Mutual Acceptance of Data; GCP, Good Clinical Practice; STED, Summary Technical Documentation; IMDRF, International Medical Device Regulators Forum; RPS-ToC, Regulated Product Submission- Table of Contents; HDE, Humanitarian Device Exemption; MA, Marketing Authorization.