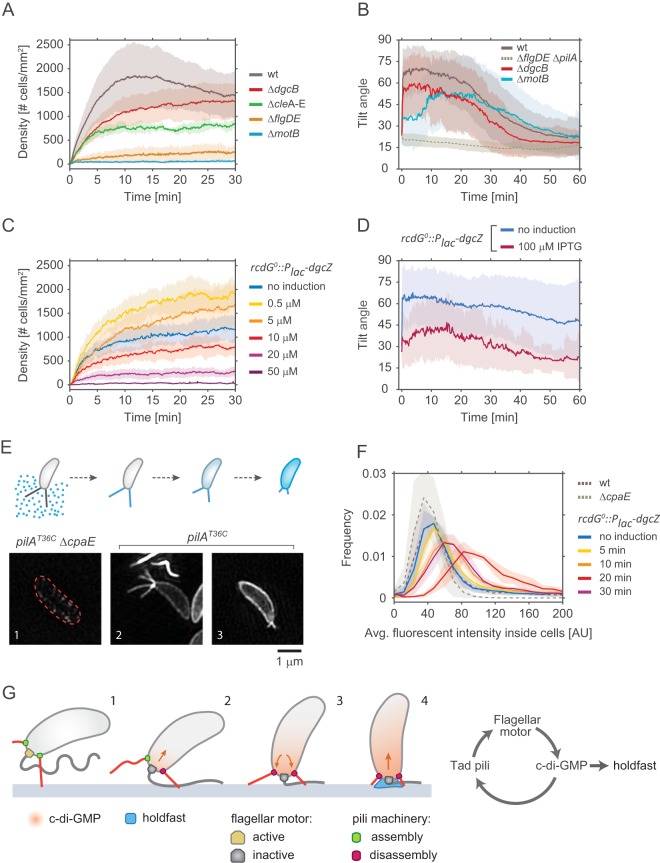
FIG 5.
Effect of c-di-GMP on pilus activity and surface attachment. (A) Pilus-mediated surface attachment in different strains of C. crescentus strain. The colonization density was determined over time in a microchannel at a constant medium flow rate of 0.75 mm/s. All strains used were defective in holdfast secretion (NA1000). Shadow areas represent standard deviations. Number of replicates: wt strain = 14, strain ΔdgcB = 14, strain ΔflgDE = 6, strain ΔmotB = 10, strain ΔcleA–E = 6. (B) Pilus-mediated standing up of SW cells. The tilt angle θ was determined in newborn SW cells of the strains indicated. Time zero corresponds to the moment of SW cell separation from its mother. Shadow areas represent standard deviations. All strains had a functional holdfast. Number of replicates: wt strain = 96, strain ΔmotB = 54, strain ΔdgcB = 34, strain ΔpilA = 15. (C) Pilus-mediated attachment efficiency as a function of the presence of or absence of c-di-GMP. Strain NA1000 rcdG0::Plac-dgcZ was grown at increasing IPTG concentrations to raise intracellular c-di-GMP and was analyzed for surface colonization as outlined for panel A. Shadow areas represent standard deviations. Number of replicates: no induction = 8, 0.5 μM = 6, 10 μM = 6, 20 μM = 4, 50 μM = 6. (D) Pilus-mediated standing up of SW cells as a function of c-di-GMP concentration. The angle θ was determined for newborn SW cells of strain rcdG0::Plac-dgcZ after cell division. Two distinct dgcZ expression levels were tested: no induction (n = 44) and 100 μM IPTG (n = 41). Shadow areas represent standard deviations. (E) The upper schematic shows the labeling process for pili. Fluorescent maleimide dye in the medium can label only exposed cysteines, such as those in elongated pili carrying mutated pilAT36C pilin subunits. When labeled pili were retracted, pilin subunits diffused in the cell membrane, and the fluorescent signal correlates with the amount of pilin subunits in the membrane. The lower part of the panel shows representative superresolution images of labeled cells. (Step 1) Strains that carry pilAT36C but that are unable to assemble pili due to a mutation in the motor machinery (ΔcpaE) do not acquire any significant fluorescence. (Step 2) In cells carrying pilAT36C and a functional machinery, elongated pili are strongly labeled. (Step 3) Upon retraction, disassembled pilin subunits reside in the membrane. (F) The chart shows the distribution of the average fluorescent signal of SW cell membranes in different strains carrying the mutation pilAT36C after labeling with AF488-mal for exact incubation time windows. (Replicates = >3; analyzed cells = >6,000). Note that because AF488-mal nonspecifically binds to holdfast material, all strains used here were devoid of holdfast. (G) Model of surface attachment and transition from temporary to long-term attachment. Within a few seconds of landing, cells sense the surface via the flagellar motor and increase their levels of intracellular c-di-GMP. In turn, c-di-GMP triggers the secretion of the holdfast and increases the rate of retraction of pili.