Abstract
We present a case of acute myeloid leukemia with der(1)t(1;19)(p13;p13.1) translocation and RUNX1 mutation. A literature review summarizing the clinical, pathological, and molecular features of the published cases is also presented.
Keywords: Acute myeloid leukemia, AML, RUNX1, Hand-mirror blasts, t(1;19)
1. Introduction
Cytogenetic abnormalities provide important prognostic information and serve as the frame work for risk adapted therapy for acute myeloid leukemia [1]. Some of the karyotypic abnormalities are sufficiently common, have been well characterized at the molecular level, and are known to describe distinct entities. For example translocations t(8;21), t(15;17), and t(16;16) are known to impart a favorable prognosis while abnormalities of chromosome 3q, monosomies of chromosome 5 and/or 7, or complex karyotypes portend an adverse prognosis [2], [3].
Up to 10% of AML patients have rare recurrent cytogenetic abnormalities [4]. Many of these have not been characterized in detail and their impact on prognosis remains unclear. We present a case of AML with der(1)t(1;19)(p13;p13.1) as the sole cytogenetic abnormality along with a concise review of the 4 previously described cases of AML with this anomaly. Next generation sequencing performed in our patient at the time of disease relapse identified a RUNX1 mutation, which has not been described in any of the previously published cases.
2. Case report
A 20-year-old Hispanic male presented with oral ulcers, fever, fatigue and headache. Prior to presenting to the emergency department, he had received a course of antibiotics for suspected sinusitis. He had developed a pruritic drug rash over his legs, buttocks and abdomen following the antibiotic use. His medical history was significant for Henoch Schoenlein purpura as a child and mild autism. No familiar history of hematologic diseases reported by the patient or otherwise documented. On examination, temperature was 38.1 °C and heart rate was 125 beats per minute. Blood pressure and respirations were normal. His physical examination showed some non-tender submandibular lymphadenopathy, left upper quadrant tenderness, and a mild maculopapular rash was seen over both legs and lower abdomen. Liver and spleen were not palpable.
Initial laboratory studies showed a leukocyte count of 0.85 × 103/µL, a hemoglobin of 5.6 g/dL, and a platelet count of 123 × 103/µL. Leukocyte differential included 2% neutrophils, 3% bands, 13% monocytes, 70% lymphocytes, and 12% blasts. Liver and renal functions were normal.
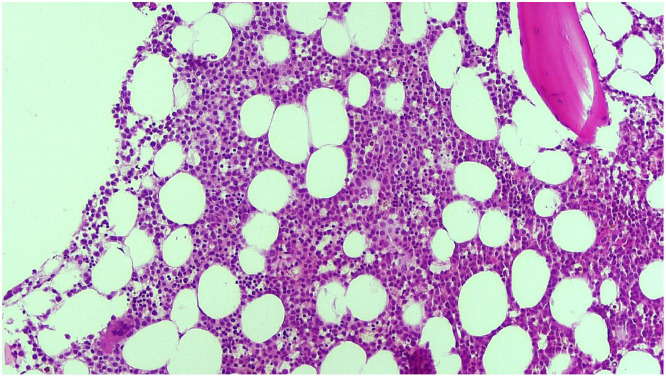
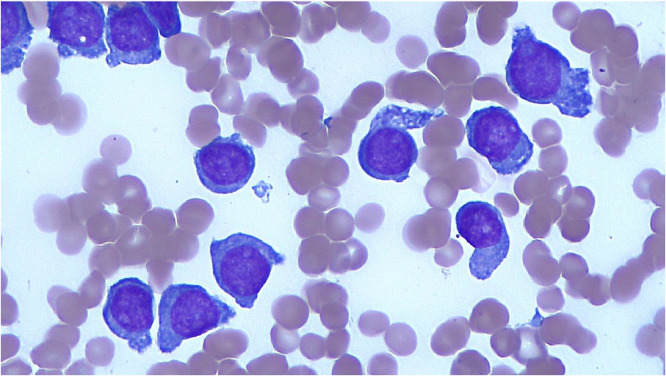
A bone marrow biopsy showed a cellularity of 70–80% with diffuse infiltration by blasts and suppression of normal hematopoiesis (Fig. 1). Blasts showed monocytic features with a variable proportion of the cells displaying “hand-mirror” morphology with pronounced cytoplasmic tails and blebs (Fig. 2). Flow cytometry revealed the blasts to be CD117(+), CD34(-), HLA-DR(+), CD4 (+) and CD64(+).
Fig. 1.
Bone marrow morphology demonstrating hypercellularity (70–80%) with infiltration of myeloid blasts and suppression of normal hematopoiesis.
Fig. 2.
Myeloid blasts with pronounced cytoplasmic tails (hand-mirror blasts).
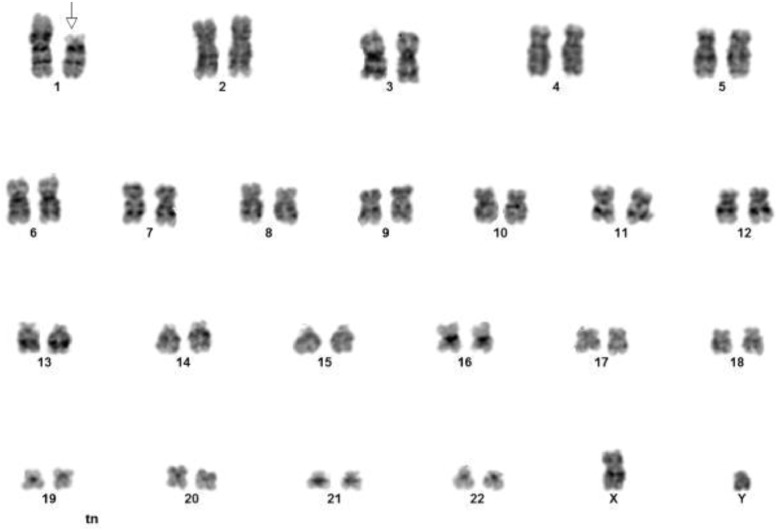
Cytogenetic analysis was performed on bone marrow aspirate. All 22 metaphases analyzed revealed the presence of a derivative chromosome 1 resulting from an unbalanced translocation of the short arm of chromosome 19 proximal to 19p13 to the short arm of chromosome 1 at band 1p13: 46, XY, der(1)t(1;19)(p13;p13.1)[22] (Fig. 3).
Fig. 3.
Cytogenetic analysis demonstrating 46, XY, der(1)t(1;19)(p13;p13.1) karyotype.
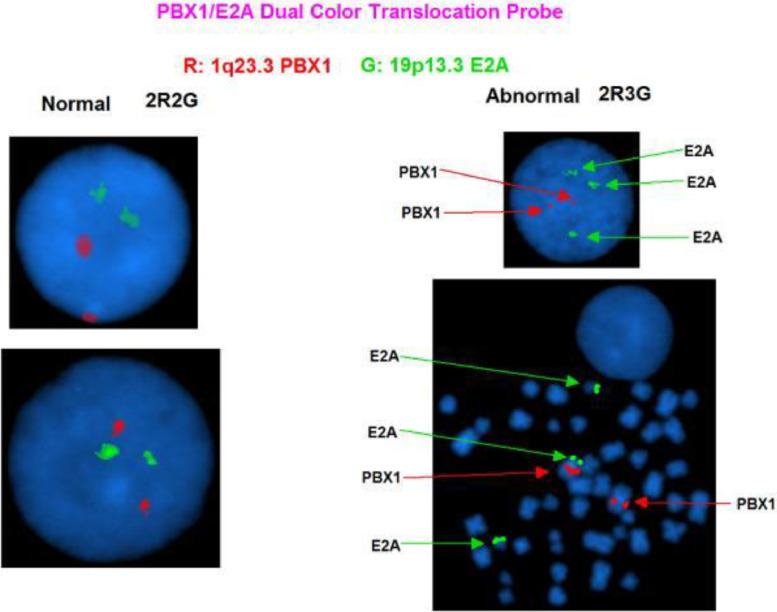
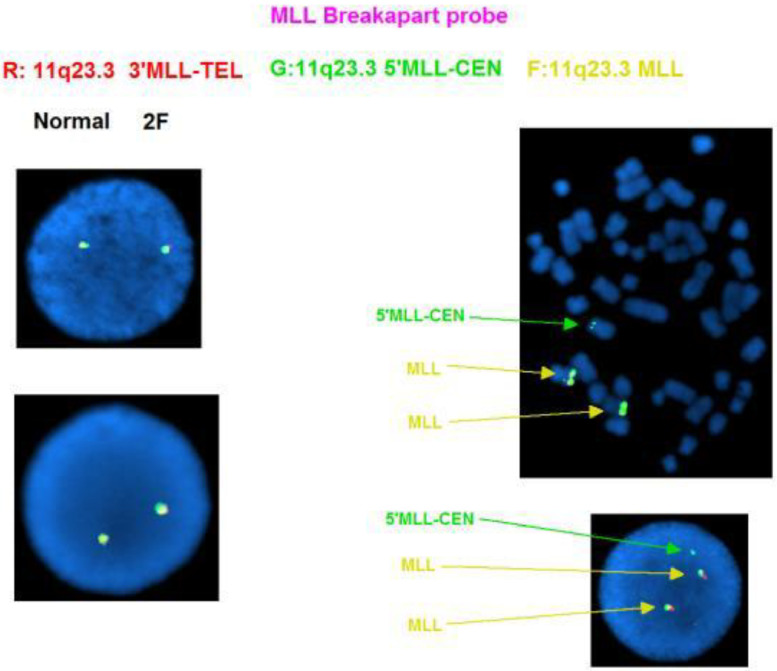
FISH (Fluorescent in-situ hybridization) analysis was performed using acute leukemia-specific probes. FISH results using the E2A/PBX1 Translocation, Dual Fusion Probe showed the translocation resulted in an extra copy of the E2A gene (19p13.3) on the derivative chromosome (Fig. 4). The E2A probe, labelled in green, contains two probes (110 kb and 146 kb) that cover the 3′ end of the E2A (TCF3) gene and flanking region and a 321 kb probe that covers a region 5′ (centromeric) to the gene. The PBX1 probe, labelled in red, contains two probes (147 kb and 110 kb) that map within the PBX1 gene and a 117 kb probe that maps 3′ (telomeric) to the gene. In a normal cell, two red and two green signals (2R, 2G) should be visible. Due to the close proximity of the green signals, these two signals may overlap and appear as one signal in a small proportion of cells. In this case, since there is gain of an extra E2A gene due to the unbalanced translocation, 2R3G signal pattern was evident by FISH (Fig. 4). DAPI images of the metaphases revealed that this extra E2A gene copy was on the short arm of chromosome 1 at band 1p13 (Fig. 4). In addition, an extra copy of the MLL (11q23.3) gene was also identified (Fig. 5).
Fig. 4.
Fluorescent in situ hybridization demonstrating unbalanced translocation of chromosome 1 band p13 and the short arm of chromosome 19 band p13 resulting in an extra copy of the E2A gene.
Fig. 5.
FISH analysis demonstrating an extra copy of the 5′MLL (11q23.3) gene. .
Molecular testing at diagnosis with FISH using extended pediatric acute lymphoblastic leukemia panels did not show mutations in FLT3 (either ITD or TKD), NPM1, CEBPA or KIT.
The patient underwent induction therapy with a typical 7 + 3 regimen (employing 200 mg/m2/day of cytarabine and 12 mg/m2 of idarubicin). Complete remission (by morphology, flow cytometry and FISH) was achieved after induction. Repeat karyotype was 46,XY[20]. The patient was advised to undergo an allogeneic stem cell transplant in 1st remission. He, however, declined and received 4 cycles of consolidation with high dose cytarabine. At the end of 4 cycles of consolidation, a repeat bone marrow aspiration and biopsy showed ongoing morphological and cytogenetic remission.
He relapsed 4 months after completing therapy. Bone marrow biopsy and aspirate again showed “hand mirror” myeloblasts with monocytic differentiation and flow cytometry again identified expression of CD 117, CD 4, and CD 64 without CD 34 expression. Cytogenetic findings at relapse, however, continued to show a normal male karyotype (46,XY[20]) and FISH studies looking specifically for E2A and MLL showed normal copy numbers.
Next generation sequencing was performed using Illumina sequencing technology to identify variants in genes commonly associated with AML (ASXL1, ATM, BCOR, BCORL1, CBL, CDKN2B,CEBPA, CREBP(ATF2), CSF3R, DDX41, DNMT3A, ETV6, EZH2, FLT3, GATA1, GATA2, IDH1, IDH2, IKZF1, JAK2, KDM6A, KIT, KRAS, MLL, MPL, NF1, NPM1,NRAS, PHF6, PTEN, PTPN11, RUNX1, SETBP1, SF3B1, SRSF2, STAG2, STK11, TET2, TP53, U2AF1, WT1, ZRSR2). The above genes, except for MLL, were tested using a commercial bait-capture next generation sequencing platform. MLL was tested by long range PCR which only detected the presence or absence of the partial tandem duplication. This revealed a missense mutation on the RUNX1 gene, c.127C > T variant ID 463,980, with a frequency of 55%, which resulted in the replacement of proline with serine at codon 43 of the RUNX1 protein (p.Pro43Ser). No other variants in the above genes were identified.
He received salvage therapy with fludarabine, cytarabine and idarubicin (FLAG-IDA) and was then treated with venetoclax and azacytidine. Second remission lasted 3 months. He then developed leukemia cutis. Repeat therapy with FLAG-IDA was attempted with no response and the patient opted for supportive care alone.
3. Discussion
We present a case of AML with der(1)t(1;19)(p13;p13.1) as the sole cytogenetic abnormality. The Mitelman database for chromosomal aberration and gene fusion, Atlas of genetics and cytogenetics in oncology and hematology, and PubMed were searched for all cases of AML with der(1)t(1;19). An additional 4 cases of acute myeloid leukemia were identified [5], [6], [7], [8]. Table 1 summarizes the clinical, pathological and molecular findings in these, including the current case. The Median age of patients is 36 years (range 1–84). Median presenting WBC count is 1100/mcl (700–265,000). There are 3 males and 2 females. Two patients had M5 blast morphology and two had “hand mirror” blasts noted on smears. In 2 patients der(1)t(1;19) was the sole cytogenetic abnormality, while the other 3 had additional chromosomal abnormalities.
Table 1.
Clinical, pathologic, and molecular characteristics of existing cases demonstrating t(1;19) translocations.
| Ref | Age (yrs) | Sex | WBC(/mcl) | Hb (g/dl) | Plt 103/mcl | Blast morphology | karyotype | Molecular mutations | prognosis |
|---|---|---|---|---|---|---|---|---|---|
| 5 | 36 | M | 700 | 5.4 | 13 | M1 with hand mirror blasts. | 46,XY, + der(1)t(1;19)- (pl3;pl3.1)[cp3]/46,XY | NR | NR |
| 6 | 1 | F | 265,000 | 7 | 68 | M5 | 47,XX,+der(1)t(1;19)(p13;p13.1) der(10)inv(10)(p2?5q25)t(10;11)(q25;q25) | NR | CNS relapse at 5 months, expired 8 months. |
| 7 | 73 | M | 1100 | 5 | 31 | 48,XY, der(1)t(1;19)(p13;p13.1), +1 3 | IDH2 C419 G > 8 FLT3ITD | Expired, 21 months | |
| 7 | 84 | F | 10,000 | 10 | 56 | 46,XX, der(1;19)(p13;p13.1), −22 | TET2 C4022C > A | Expired, 1 month | |
| SF3B1 c.2359_2360delinsT, c.362_363delinsA | |||||||||
| FLT3ITD | |||||||||
| KRAS c.183A > C | |||||||||
| NPM1 c.859delinsCTCTG | |||||||||
| Curr-ent | 21 | M | 850 | 5.6 | 123 | M5 hand mirror blasts | 46, XY,der(1)t(1;19)(p13;p13.1) | RUNX1p43s | Relapse 9 months. |
Of the 3 cases where molecular findings were reported (including the current one), 2 had FLT3 ITD mutations; a RUNX1 mutation was found in our case. Our case is unique in being the first to describe the karyotype both at presentation and at relapse and being the first to identify the presence of RUNX1 mutation in association with this karyotype.
RUNX1 encodes for a transcription factor which is essential for hematopoiesis. Point mutations in RUNX1 in acute leukemia were first described in 1999. It is now evident that these are frequent in acute myeloid leukemia being present in up to 33% of AML with normal karyotype or non-complex chromosomal imbalances. Their presence is an independent adverse prognostic factor [9], [10], [11]. The current (2016) revision of WHO classification of myeloid neoplasms includes a provisional entity entitled AML with mutated RUNX1 [12], [13]. In our case, the lack of RUNX1 mutational testing performed on the initial sample prevent us from excluding acquisition of the mutation at the time of disease relapse when the presence of the abnormal chromosome clone and derivative chromosome were no longer detected. Although there was no familiar history of leukemic disorders in this patient, in view of his young age and high allele burden (55%), the possibility of a constitutional RUNX1 mutation could not be entirely excluded. We conclude that presence of der(1)t(1;19) in AML marks the presence of adverse molecular mutations and poor prognosis. Molecular findings in future cases need to be studied both at presentation and relapse to better understand the biology of this leukemia and improve outcomes.
Acknowledgment
We would like to thank Weidong Zhou, MD and Michael Van Ness, MD at Quest Diagnostics for their assistance with the next generation sequencing result interpretation.
References
- 1.Grimwade D. Impact of cytogenetics on clinical outcome in AML. In: Karp J.E., editor. Acute Myelogenous Leukemia. Humana Press; Totowa, NJ: 2007. pp. 177–192. editor. [Google Scholar]
- 2.Mrózek K., Heerema N.A., Bloomfield C.D. Cytogenetics in acute leukemia. Blood Rev. 2004;18(2):115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 3.Grimwade D., Hills R.K.Independent prognostic factors for AML outcome. Hematology Hematol. Am. Soc. Hematol. Educ. Program2009:385–95. [DOI] [PubMed]
- 4.Grimwade D., Hills R.K., Moorman A.V. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 5.“NM_001754.4(RUNX1):C.127C>T (P.Pro43Ser) Variation Report - ClinVar - SCV000638125.2.” National Center for Biotechnology Information, U.S. National Library of Medicine, www.ncbi.nlm.nih.gov/clinvar/variation/463980/.
- 6.Ma S.K., Wan T.S.K., Chan L.C., Chiu E.K.W. Hand-mirror blasts, AML-M1, and der(1)t(1;19)-(p13;p13.1) Leuk. Res. 2000;24:95–96. doi: 10.1016/s0145-2126(99)00154-x. [DOI] [PubMed] [Google Scholar]
- 7.Tchinda Joëlle. Novel Der(1)t(1;19) in two patients with myeloid neoplasias. Cancer Genet. Cytogenet. 2002;133(1):61–65. doi: 10.1016/s0165-4608(01)00505-2. [DOI] [PubMed] [Google Scholar]
- 8.Salgado R.N., Menezes J., Calvente M. Myeloid neoplasms with der(1)t(1;19) may constitute a specific entity characterized by a cytogenetic biomarker and gene mutations involved in DNA methylation. Leuk. Lymphoma. 2014;55(11):2652–2655. doi: 10.3109/10428194.2014.891024. [DOI] [PubMed] [Google Scholar]
- 9.E2A/PBX1 Translocation, Dual Fusion Probe Kit insert; Cytocell; DS230/CE v004.00/2018-01-31.
- 10.Schnittger S., Dicker F., Kern W. RUNX1 mutations are frequent in de novo AML with noncomplex karyotype and confer an unfavorable prognosis. Blood. 2011;117(8):2348–2357. doi: 10.1182/blood-2009-11-255976. [DOI] [PubMed] [Google Scholar]
- 11.Tang J.L., Hou H.A., Chen C.Y. AML1/RUNX1 mutations in 470 adult patients with de novo acute myeloid leukemia: prognostic implication and interaction with other gene alterations. Blood. 2009;114(26):5352–5361. doi: 10.1182/blood-2009-05-223784. [DOI] [PubMed] [Google Scholar]
- 12.Gaidzik V.I., Bullinger L., Schlenk R.F. RUNX1 mutations in acute myeloid leukemia: results from a comprehensive genetic and clinical analysis from the AML study group. J. Clin. Oncol. 2011;29(10):1364–1372. doi: 10.1200/JCO.2010.30.7926. [DOI] [PubMed] [Google Scholar]
- 13.Arber D.A., Orazi A., Hasserjian R. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]