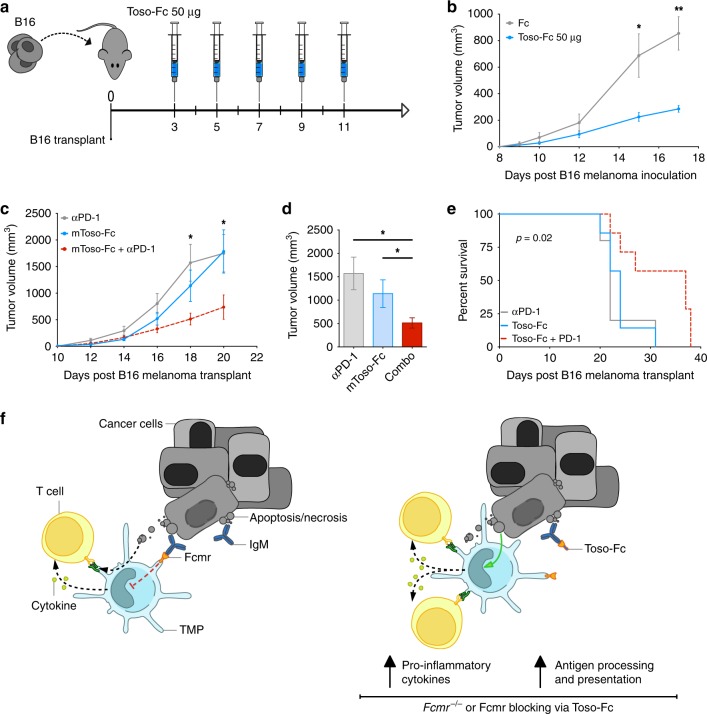
Fig. 6.
Toso-Fc reduces tumorigenesis when combined with anti-PD-1. a Schematic diagram of the experiment showing the ventral–lateral transplantation of B16F0 cells superior to the inguinal LN via intradermal injection into Fcmr+/+ recipient mice (left), and the associated timeline of treatment with Toso-Fc fusion protein decoy receptor starting 3 days post-transplant (right). b Time course of tumor growth in the Fcmr+/+ mice receiving Toso-Fc or Fc control treatment as illustrated in (a) (n = 10 mice/group). Data are representative of three separate experiments. c Time course of tumor growth in Fcmr+/+ mice transplanted as in (a) and receiving either anti-PD-1 antibody or Toso-Fc alone, or in combination, starting at 6 days post-B16 cell transplant (n = 8 mice/group). d Quantification of tumor volumes in the mice in (c) at 18 days post-B16 cell transplant. e Survival curves for the mice in (c). f Schematic diagram illustrating the functional implications of Fcmr ligation in MPs within the TME. Left: Fcmr–ligand interactions normally limit antigen processing/presentation and pro-inflammatory cytokine production by MPs, resulting in reduced T cell activation and decreased anti-tumor immune responses. Right: The Toso-Fc decoy receptor is hypothesized to out-compete Fcmr for binding to its ligand, preventing Fcmr–ligand interaction and thus removing the restraints on MP activation. The resulting increases in antigen processing/presentation and pro-inflammatory cytokine production promote T cell-mediated anti-tumor immunity. Toso-Fc is thus a potentially valuable immunotherapeutic agent. Data are represented as mean ± SEM (ANOVA t test; *p < 0.05; **p < 0.01; ***p < 0.001)