Abstract
Introduction
Three-dimensional (3D) multicellular spheroids are useful tools for simulation of cellular functions in vitro. However, it is difficult to culture certain epithelial cell types under 3D spheroid conditions because these cells cannot resist autonomous cell death, triggered by disordered cell polarity. The objective of this study was to find a method that enables spheroid culture of such epithelial cells utilizing hydrogel beads without cell death.
Methods
We used murine E14.5 fetal hepatic cells for the spheroid composition because they are sensitive to disorganized structures. Spheroids were formed by injecting 1-μl fresh medium containing 1000 fetal hepatic cells and the same number of alginate hydrogel beads (20 μm in diameter) into a 3% methylcellulose medium in the presence of dexamethasone and oncostatin M to induce hepatic differentiation. After 7 days of culture, microstructures were observed using hematoxylin and eosin staining and immunostaining using anti-CK8/18 antibody. Albumin secretion rate was determined by the enzyme-linked immunosorbent assay method. In addition, polarity-related proteins, E-cadherin, ezrin, and MRP2 were observed with immunostaining.
Results
Control spheroids without the use of alginate hydrogel beads showed extensive internal lack of epithelial hepatic cells. The spheroids containing alginate hydrogel beads exhibited sheet- or cord-like structures of epithelial hepatic cells, and it was clear that cell death of epithelial cells had been prevented. Albumin secretion data also supported the improvement of epithelial hepatic cell viability when alginate hydrogel beads were used. Localization of polarity-related proteins indicated the partial reconstitution of cell polarity in the spheroids using alginate hydrogel beads.
Conclusion
Based on these data, we concluded that the application of alginate hydrogel beads was effective in improving the epithelial hepatic cell culture of multicellular spheroids.
Keywords: Spheroid, Fetal hepatic cell, Alginate hydrogel bead, Cell polarity, Microstructure
Abbreviations: 3D, three-dimensional; MC, methylcellulose; HE, hematoxylin and eosin; CK, cytokeratin; MRP, multidrug resistance-associated protein; ELISA, enzyme-linked immunosorbent assay
Highlights
-
•
It was difficult to maintain epithelial fetal hepatic cells in their multicellular spheroids.
-
•
The difficulty to maintain the fetal hepatic cells was overcome by embedding of alginate hydrogel beads into the spheroids.
-
•
Albumin secretion was improved by embedding of the alginate hydrogel beads.
-
•
Cell polarity was partially established in the hybrid spheroids.
1. Introduction
Multicellular spheroids are important for cell-based assays because cellular functions are highly enhanced in the three-dimensional (3D) culture. However, necrosis in the central areas of the relatively larger multicellular spheroids can sometimes occur because of oxygen/nutrient limitation [1], [2], [3], [4], [5], [6], and this necrosis causes a decline in the spheroid functions. In addition, epithelial cells can sometimes form “multicellular cysts” through the induction of apoptosis; this is because epithelial cells tend to form polarized monolayer structures instead of disorganized structures [7]. This event arising independently from oxygen/nutrient limitation is also at risk for diminishing of the spheroid functions. We previously published a method of embedding alginate hydrogel beads into spheroids to improve oxygen/nutrient supply [8]. In this study, we show that the method is also critical in preventing loss of epithelial cells when multicellular spheroids are formed with fetal hepatic cells. We attempt to clarify the formation of cell polarity in such spheroids by visualizing polarity-related proteins.
2. Materials and methods
2.1. Fetal hepatic cells
Pregnant C57BL/6NCrSlc mice were obtained from Japan SLC (Hamamatsu, Japan), and fetal hepatic cells were isolated by collagenase digestion [9]. In brief, the E14.5 fetal livers were minced and enzymatically digested with Liver Digest Medium (17703-034, Waltham, MA Thermo Fisher Scientific). After the hemolysis of red blood cells, the hepatic cells were purified by centrifugation. Isolated cells were suspended in the Dulbecco's Modified Eagle's Medium (041-29775, Wako, Osaka, Japan) supplemented with 10% fetal bovine serum (Cellgro, 35-010-CV, CORNING, Corning, NY, USA), 2 mmol/l GlutaMAX (35050-061, Thermo Fisher Scientific), 1× MEM Non-Essential Amino Acid Solution (11140-050, Thermo Fisher Scientific), 1× Insulin-Transferrin-Selenium X Supplement (51500-056, Thermo Fisher Scientific), 50 μg/ml gentamycin (15710-064, Thermo Fisher Scientific).
2.2. Preparation of alginate hydrogel beads
Alginate hydrogel beads were obtained as reported in a previous study [8]. Tiny droplets of 1.5% w/v sodium alginate (Sigma–Aldrich, St. Louis, MO, USA) solution were discharged from an inkjet system (Cluster Technologies, Osaka, Japan) into a reservoir flask filled with 5% calcium chloride (Wako, Osaka Japan) solution. The droplets of the alginate solution change into a gel immediately after they have been dropped into a solution containing calcium ions. When a 25-μm diameter nozzle was used, the size of the droplet was approximately 20 μm. Alginate hydrogel beads were washed with phosphate-buffered saline and suspended in fresh culture medium.
2.3. Fabrication of hybrid spheroids
The aggregation method used to fabricate hybrid spheroids was previously reported [10]. To make the 3% methylcellulose (MC) medium, 3 g MC (viscosity, 4000 cP; M0512; Sigma–Aldrich) was sterilized by autoclaving in a bottle. To MC, 100 ml of the culture medium was added; it was dispersed by mixing with a magnetic stirrer on ice. The MC medium was poured into 35-mm petri dishes with a positive-displacement pipette (Microman; Gilson, Middleton, WI, USA). The cells and alginate hydrogel beads were suspended in the normal culture medium, and the density of cells and beads were both adjusted to 1 × 106 cells/ml. The injection of 1 μl of the normal medium with cells and beads into the MC medium resulted in the rapid aggregation of both cells and beads. Because the aggregates did not sink to the bottom of the petri dish, they were able to be cultured for 7 days in the MC medium without adhesion to the bottom. To prevent dessication, 250 μl of culture medium was added to the MC medium at day 3. Throughout the culture period of 7 days, 10−7-M dexamethasone (041-18861, Wako) and 10 ng/ml-oncostatin M (495-MO-025, Bio-techne, Minneapolis, MN, USA) were also added to the MC medium for inducing hepatic maturation [9]. To remove spheroids from the MC medium, 5 U/ml-cellulase solution (Onozuka RS; Yakult Pharmaceutical Industry, Tokyo, Japan) was added to the MC medium, and the mixture was incubated for 30 min at 37 °C to digest MC. After this step, spheroids were easily collected in a test tube.
2.4. Staining of spheroid sections
Paraffin-embedded spheroids were sectioned and visualized by hematoxylin and eosin (HE) staining or immunohistochemistry. For the detection of specific proteins, several antibodies were used as a primary antibody that included the following: anti-cytokeratin (CK) 8/18 (GP11; PROGEN Biotechnik, Heidelberg, Germany); anti-E-cadherin (610181; Becton, Dickinson and Company, Franklin Lakes, NJ, USA); anti-multidrug resistance-associated protein (MRP) 2 (sc-5770; Santa Cruz Biotechnology, Dallas, TX, USA); and anti-ezrin (sc-58758; Santa Cruz).
2.5. Albumin secretion assay
After 7 days culture in the MC medium, multicellular spheroids with or without alginate hydrogel beads were removed from the MC medium by decreasing the viscosity of the medium and transferring spheroids to untreated 12 well plates with fresh culture medium. The spheroids were then incubated, and the medium was sampled at 0 h and 48 h. Albumin secreted into the medium was detected using an enzyme-linked immunosorbent assay (ELISA) kit (Shibayagi, Gunma, Japan). At the end of the assay, genomic DNA was extracted from the spheroids and quantified using a fluorometer (Quantus; Promega, Madison, WI, USA) to normalize albumin secretion by cell number. All values were expressed as mean ± SD based on three samples. Student t-tests were used to compare the samples, and differences were considered significant at p < 0.05.
3. Results and discussion
3.1. Prevention of loss of epithelial fetal hepatic cell in the multicellular spheroids
Primary fetal hepatic cells isolated from an E14.5 mouse were injected into the MC medium and cultured for 7 days in the presence of hepatic differentiation inducers, dexamethasone, and oncostatin M. Spheroids at day 7 were sectioned and observed by HE staining or immunohistochemistry. Fig. 1a shows that inside of the spheroid was healthy and there were cells on the surface of the spheroid showing darker pink color. Based on our previous study, fetal hepatic cells were mainly composed of fetal hepatocytes those are CK8/18, a marker for epithelial cells, positive [11]. However, Fig. 1c shows that CK8/18 positive cells were detected only the surface of the spheroid. In contrast, spheroids obtained by injecting both primary fetal hepatic cells and alginate hydrogel beads showed microchannel-like structures, and large amount of cells displayed darker pink color and they were also CK8/18 positive (Fig. 1b, d).
Fig. 1.
Inner structure of multicellular spheroids comprising fetal hepatic cells. After 7 days culture, the spheroids in the MC medium were fixed, embedded in paraffin, and sectioned. (a and b) HE staining was performed to observe the inner structures. (c and d) CK8/18 was visualized by a fluorescent immunostaining method. a and c show cellular aggregates without beads, and b and d show cellular aggregates with beads. Bar: (a, b) 100 μm, (c, d) 50 μm.
CK8/18 staining clearly shows that the cell populations between two conditions seemed completely different. The reason for the difference is not clear but it is certain that CK8/18 positive cells diminished in the control condition. One possibility is that epithelial cells tend to form single layered cyst structure in the 3D multicellular spheroids by apoptosis [7]. In this case, other types of cells like mesenchymal cells might grow inside of the cyst-like structure formed by epithelial cells. This is because the fetal hepatic cells we used in this study were crude and not pure epithelial hepatocytes. The inner structures of the hybrid multicellular spheroids were composed of layers of cell-sheets/cords and void spaces formed by the alginate hydrogel beads. We believed that the hepatic cells were able to survive because they formed sheet- or cord-like structures, enabling the survival of epithelial hepatic cells. The method to form hybrid spheroids using alginate hydrogel beads should be applicable to various epithelial cell types and mitigate disorganized structures in spheroids.
3.2. Improvement of albumin secretion
Next, we tried to confirm whether the function was improved in the hybrid spheroids. The aggregates cultured for 7 days were transferred to fresh culture medium and incubated for 48 h. Albumin concentration and DNA quantity were measured to calculate the albumin secretion rate normalized by cell number. Fig. 2 shows that the albumin secretion rate was higher in the hybrid spheroid conditions, indicating that multicellular spheroids using alginate hydrogel beads were able to maintain cell survival.
Fig. 2.
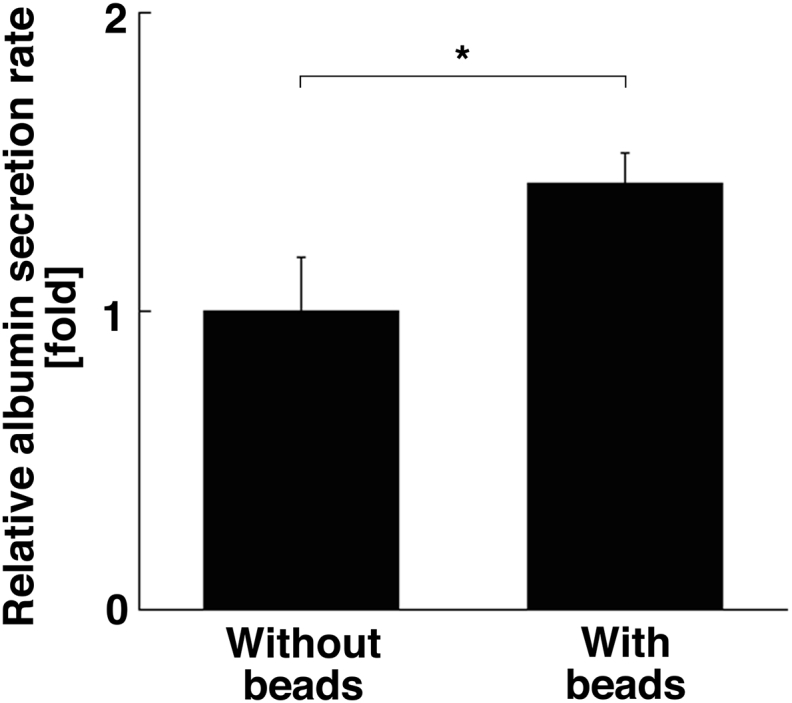
Relative albumin secretion rates. After 7 days culture, the spheroids were isolated from the MC medium and transferred to normal culture medium to measure albumin secretion. Sampling was performed at 0 and 48 h, and the supernatants were applied to ELISA. Genomic DNA was extracted and measured to normalize the albumin secretion by cell number. Data were shown as relative values, which were normalized by the rate observed for the condition “without beads”. Data represent mean ± SD. *p < 0.05.
A key difference of albumin function between normal and hybrid conditions is not only the number of epithelial fetal hepatic cells but also their condition. Sheet- or cord-like structures in the hybrid spheroid may provide better culture conditions for the epithelial hepatic cells, because the cell shape illustrated by CK8/18 staining in the hybrid spheroid (Fig. 1d) was polygonal and larger than control spheroids (Fig. 1c).
3.3. Expression of cell polarity-related proteins
The localization of cell polarity-related proteins was subsequently evaluated in the hybrid spheroids because we believed that sheet- or cord-like structures help to acquire cell polarity. E-cadherin, a homophilic cell-to-cell adhesion protein [12], was recruited to the area between cell-to-cell adhesions, showing that a lateral compartment was established in the fetal hepatic cell spheroids using alginate hydrogel beads (Fig. 3b). When alginate hydrogel beads were not used, we could not detect the localization of E-cadherin (Fig. 3a). Ezrin, an apical compartment-related protein [13], was detected at in the hybrid spheroid conditions with some localization (Fig. 3d). Ezrin expression was not detected at any level in spheroids without alginate hydrogel beads (Fig. 3c). We also confirmed the expression and localization of the transporter protein MRP2 [14] in the hybrid spheroids. MRP2 expression in the normal spheroid was not detectable at day 7 (Fig. 3e). In contrast, MRP2 was easily detectable in the cytosol area in the hybrid spheroids (Fig. 3f). However, the transporter was not localized at the apical area. The difference between partly localized ezrin and non-localized MRP2 was also observed when data were merged (Fig. 3h).
Fig. 3.
Immunostaining of polarity related proteins. Multicellular spheroids were sectioned and stained with (a, b) anti-E-cadherin, (c, d) anti-ezrin, and (e, f) anti-MRP2; g and h are merged panels of both c and e and d and f, respectively. The arrow heads labeled h indicate localized signals of ezrin protein. a, c, e, and g represent the conditions without alginate hydrogel beads, while b, d, f, and h represent the results from culture with beads. Bar: 100 μm.
These results indicate that cell polarity was partially established in the hybrid spheroid; MRP2, one of the key molecules for drug elimination, was not recruited to the apical location of cells. However, even partial establishment of cell polarity, it was enough to prevent loss of epithelial fetal hepatic cell (Fig. 1, Fig. 3). The establishment of complete cell polarity in 3D tissue in vitro using hepatocytes remains a big challenge for use in drug screening and regenerative medicine. Additional studies are still needed to recapitulate complete cell polarity, including apical, lateral, and basal domains. However, our strategy to regulate cell alignment in 3D multicellular spheroids should help to accomplish these goals of screening and regenerative medicine.
4. Conclusion
Our method for making hybrid spheroids comprising cells and alginate hydrogel beads was useful in maintaining epithelial hepatic cells and their function in 3D multicellular spheroids. Partial cell polarity was acquired using this method employing epithelial fetal hepatic cells cultured for 7 days in the presence of hepatic differentiation reagents.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
This work was partly supported by Grant-in-Aid for Scientific Research on Innovative Areas “Bio Assembler” Grant number 26106722 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Wakako Motoyama, Email: n155279f@yokohama-cu.ac.jp.
Kanae Sayo, Email: n155264e@yokohama-cu.ac.jp.
Hirotaka Mihara, Email: i130613a@yokohama-cu.ac.jp.
Shigehisa Aoki, Email: aokis@cc.saga-u.ac.jp.
Nobuhiko Kojima, Email: nobuhiko@yokohama-cu.ac.jp.
References
- 1.Mueller-Klieser W. Method for the determination of oxygen consumption rates and diffusion coefficients in multicellular spheroids. Biophys J. 1984;46:343–348. doi: 10.1016/S0006-3495(84)84030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freyer J.P., Sutherland R.M. Regulation of growth saturation and development of necrosis in EMT6/Ro multicellular spheroids by the glucose and oxygen supply. Cancer Res. 1986;46:3504–3512. [PubMed] [Google Scholar]
- 3.Sutherland R.M., Sordat B., Bamat J., Gabbert H., Bourrat B., Mueller-Klieser W. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 1986;46:5320–5329. [PubMed] [Google Scholar]
- 4.Acker H., Carlsson J., Mueller-Klieser W., Sutherland R.M. Comparative pO2 measurements in cell spheroids cultured with different techniques. Br J Cancer. 1987;56:325–327. doi: 10.1038/bjc.1987.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groebe K., Mueller-Klieser W. On the relation between size of necrosis and diameter of tumor spheroids. Int J Radiat Oncol Biol Phys. 1996;34:395–401. doi: 10.1016/0360-3016(95)02065-9. [DOI] [PubMed] [Google Scholar]
- 6.Glicklis R., Merchuk J.C., Cohen S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnol Bioeng. 2004;86:672–680. doi: 10.1002/bit.20086. [DOI] [PubMed] [Google Scholar]
- 7.Lin H.H., Yang T.P., Jiang S.T., Yang H.Y., Tang M.J. Bcl-2 overexpression prevents apoptosis-induced Madin-Darby canine kidney simple epithelial cyst formation. Kidney Int. 1999;55:168–178. doi: 10.1046/j.1523-1755.1999.00249.x. [DOI] [PubMed] [Google Scholar]
- 8.Kojima N., Takeuchi S., Sakai Y. Fabrication of microchannel networks in multicellular spheroids. Sens Actuators B Chem. 2014;198:249–254. [Google Scholar]
- 9.Kamiya A., Kinoshita T., Ito Y., Matsui T., Morikawa Y., Senba E. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999;18:2127–2136. doi: 10.1093/emboj/18.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima N., Takeuchi S., Sakai Y. Rapid aggregation of heterogeneous cells and multiple-sized microspheres in methylcellulose medium. Biomaterials. 2012;33:4508–4514. doi: 10.1016/j.biomaterials.2012.02.065. [DOI] [PubMed] [Google Scholar]
- 11.Kojima N., Sakai Y. Control of liver tissue reconstitution in mesenteric leaves: the effect of preculture on mouse hepatic progenitor cells prior to transplantation. J Robot Mechatron. 2013;25:698–704. [Google Scholar]
- 12.Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 13.Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989;108:921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchler M., Konig J., Brom M., Kartenbeck J., Spring H., Horie T. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J Biol Chem. 1996;271:15091–15098. doi: 10.1074/jbc.271.25.15091. [DOI] [PubMed] [Google Scholar]




