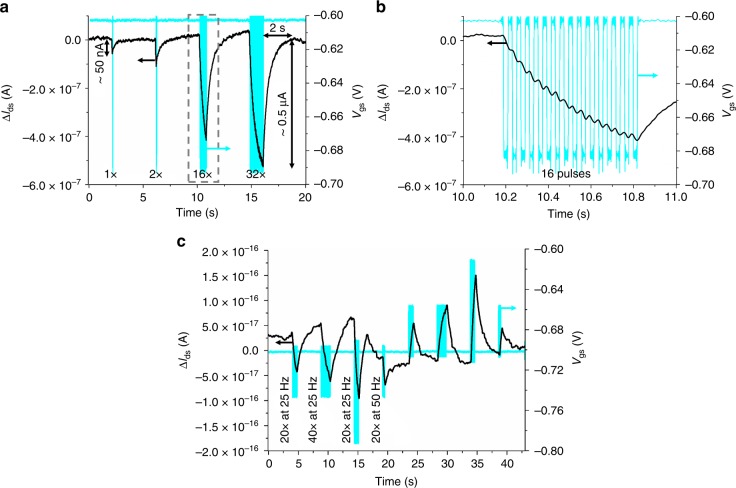
Fig. 4.
Synaptic behavior of the inkjet (IJ)-printed stretchable field effect transistors (FETs). a Source–drain current variation (ΔIds, on the left axis) response over time to small gate voltage pulses (Vgs, on the right axis) that imitates neuron presynaptic potential spikes for FETs with W/L = 1000 µm/50 µm. The pulses consist of a square signal of −80 mV of amplitude (on top of an initial biasing voltage of −0.6 V applied for long enough time to ensure the full formation of the channel), 25 Hz of frequency, and duty cycle of 50%. The drain–source voltage was held at −1.1 V. The source–drain current response is tested for consecutive trains of 1, 2, 16 (magnified in b) and 32 gate voltage pulses, displaying typical postsynaptic current variation that increases with the number of pulses and relaxes in their absence (the higher the number of pulses, the longer the relaxation time). b Zoom-in on the part of the signal delimited by the dashed box in a. The ripple in ΔIds observed for each individual pulse of only −80 mV highlights the good voltage resolution of the devices. c Source–drain current variation (ΔIds, on the left axis) over time for the same devices when voltage pulse trains are applied to the gate. The pulse trains have a duty cycle of 50% and are inputted in such a way that each train differs from the first one (control) in either: (1) number of pulses (40 pulses for the 2nd train vs. 20 for the control), (2) amplitude (−80 mV for 3rd train vs. −40 mV for control), or (3) frequency (50 Hz for the 4th train vs. 25 HZ for control). The source–drain current responds to all three factors in agreement with short-term synaptic plasticity, that is, the higher the number of pulses, their amplitude and their period, the higher the produced current variation and the longer the time needed for the devices to relax back to equilibrium. A second set of pulse trains with opposite voltage sign generates also an opposite signed current change, demonstrating that the devices are capable of both symmetrical excitatory and inhibitory postsynaptic current