Abstract
The pressing need for protein supply growth gives rise to alternative protein sources, such as insect proteins. Commercial cricket and mealworm powders were examined for their protein quality, surface charge and functional attributes. Both insect powders had similar proximate compositions with protein and ash contents of ~ 66% db (dry weight basis) and 5% db, respectively, however cricket powder contained more lipid (16.1%, db) than mealworm powder (13.7%, db). Mealworm protein had an amino acid score of 0.71 and was first limiting in lysine, whereas cricket protein was first limiting in tryptophan with an amino acid score of 0.85. In vitro protein digestibility values of 75.7% and 76.2%, and in vitro protein digestibility corrected amino acid scores of 0.54 and 0.65, were obtained for mealworm and cricket powders, respectively. Zeta potential measurements gave isoelectric points near pH 3.9 for both insect powders. Mealworm and cricket powders had water hydration capacities of 1.62 g/g and 1.76 g/g, respectively, and oil holding capacities of 1.58 g/g and 1.42 g/g, respectively. Both insect proteins had low solubility (22–30%) at all pHs (3.0, 5.0, and 7.0) measured. Cricket powder had a foaming capacity of 82% and foam stability of 86%, whereas mealworm powder was non-foaming. Values for commercial pea and faba bean protein concentrates were reported for comparative purposes. The insect proteins had similar protein quality as the pulse proteins and had higher solubility at pH 5.0 but were much less soluble at pH 7.0.
Keywords: Cricket protein, Mealworm protein, Insects, Pulse protein, Protein quality, Functionality
Introduction
Based on the projected global population growth of 9.8 billion people by 2050, more environmentally sustainable proteins sources are being sought to meet the protein demand in both developing and developed countries (FAO 2013; Henchion et al. 2017). One approach by the global community, includes exploring the potential of emerging protein sources. Insects are one of such sustainable high quality protein sources. They have a low ecological footprint (FAO 2013) due to their high feed conversion ratio (Nakagaki and Defoliart 1991), and lower water usage and greenhouse gas production relative to animal protein production (Oonincx et al. 2010). Although there are societies that commonly consume insects, mass-rearing systems are now bringing insects to a wider market that did not previously consider insects as a food source (FAO 2013). Many consumers have started to realize the environmental impact of the food they consume, especially the impacts from animal rearing. They are looking for a meat substitute, and are now more likely to adopt insects as a food choice (Verbeke 2015). A powder or flour, for use as a food ingredient, represents a more appealing way of consuming insects as opposed to consuming the insects whole (Schösler et al. 2012; Hartmann et al. 2015), but information on the physicochemical properties (e.g., composition, surface characteristics and functional properties) of ground insect flours and meals is needed before their use can occur in a wide array of food applications.
Insects are composed of high-quality protein, lipids including essential fatty acids, and fibre, as well as vitamins and minerals, although the amount of each of these components varies widely among species as well as within species due to insect diet, harvest technique, and processing (Finke 2002; FAO 2013). From a food labelling perspective mealworm is reported to be high in protein, high in magnesium, and a source of vitamin B6 and B12, riboflavin, niacin, folate and zinc (Nowak et al. 2016). Additionally, consumption of certain insect species may confer health benefits such as improved gut health, which is proposed to be due to their chitin content (Stull et al. 2018). Protein ingredients (enriched flours, concentrates, isolates) are used in a wide variety of applications (baked goods, meat or dairy replacers, sauces, snacks etc.) based on their functional and nutritional properties. Mealworm has been successfully incorporated into extruded cereal products at a 10% inclusion level (Azzollini et al. 2018). Cuj-Laines et al. (2018) also used extrusion for producing an insect enriched product by adding grasshopper meal to maize-based ready-to-eat snacks. Defatted and acid hydrolyzed mealworm and silkworm pupae flours were utilized in emulsion sausages by replacing 10% lean pork (Kim et al. 2016). Mealworm, larvae of black soldier fly and cricket flours replaced 5% wheat flour in bakery products, resulting in breads with a higher protein and fibre content (González et al. 2018). In all the aforementioned examples inclusion levels were quite low indicating there are still functionality challenges to overcome which may include selection of a specific species for a targeted functional performance.
For insects to be better utilized as protein ingredients in the food industry and gain consumer acceptance, insight into their functional behaviour and protein quality is needed. The overall goal of this study was to evaluate the protein quality (amino acid profile and in vitro digestibility) and physicochemical properties of commercial cricket (Gryllodes sigillatus) and mealworm (Tenebrio molitor) powders. Commercial air-classified yellow pea and faba bean protein concentrates were evaluated for comparative purposes as pulses, along with insects, have been classified as emerging protein sources and therefore insect protein products will share a portion of the same target market segments (Henchion et al. 2017). Novelty in this study resides with the direct comparisons between commercial insect and pulse protein ingredients to better reflect their commercial potential. The study assesses both ingredient functionality and digestibility using the same methodology across the commercial ingredients. Findings will aid in product development purposes, as new ingredient formulations are developed.
Materials and methods
Materials
Cricket (Gryllodes sigillatus) and mealworm (Tenebrio molitor) powders were purchased from Entomo Farms (Norwood, Canada); insects were fasted for 24 h before harvest, and roasted at approx. 107 °C before grinding into a powder. AGT Food and Ingredients (Saskatoon, Canada) kindly donated yellow pea (Pisum sativum) and faba bean (Vicia faba) protein concentrates produced from their respective flours through air-classification. All enzymes were purchased from Sigma Aldrich (Millipore Sigma., Burlington, USA) whereas all other chemicals used were of reagent grade and purchased from Fisher Scientific (Thermo Scientific, Waltham, USA). All water used in this study was produced using a Millipore Milli-Q™ water purification system (Millipore Sigma., Burlington, USA). Moisture, protein, ash and crude lipid contents were determined according to the AOAC (2005) methods 925.10, 920.87 (% N × 6.25), 923.03 and 922.06, respectively.
Osborne solubility fractions
Protein fractions were determined based on their Osborne solubility. One gram of each sample was dispersed in 25 mL of solvent which was as follows: albumins, ddH2O at pH 6.0; globulins, 0.5 M NaCl; prolamins, 70% (v/v) ethanol. Solutions were stirred for 30 min at room temperature then centrifuged (VWR clinical centrifuge 200, VWR International, Mississauga, Canada) at 3070×g for 10 min at room temperature. Glutelin was determined after extraction of albumin and globulin by dissolving the pellet in 0.1 M NaOH. The protein content of the supernatants was determined using micro-Kjeldahl digestion and distillation units (model 6030000, micro-Kjeldahl digestor; and Rapid Distillation Glassware, Labconco, Kansas City, USA) and divided by the amount of protein in the sample. Protein fractions were reported as percent protein of combined total protein recovered from all fractions.
Amino acid analysis
Amino acid content of the four samples was determined by POS Bio-Sciences Inc. (Saskatoon, Canada). The samples were subjected to acid/heat hydrolysis. In brief, 20 mg of each sample were weighed into separate 20 × 150 mm screw cap Pyrex® tubes containing 15 mL of 6 M HCl. Following flushing with N2 gas the tubes were capped and heated at 110 °C for 20 h. Each individual amino acid was quantified by high performance liquid chromatography using the Pico Tag amino acid analysis system (Waters Corporation, Milford, USA) (White et al. 1986; Landry and Delhaye 1994). The score of each essential amino acid was calculated as the concentration of the amino acid in mg/g protein relative to the same amino acid (in mg/g protein) in the FAO/WHO recommended protein reference pattern (FAO/WHO 1991). The amino acid values in the reference protein were as follows (amino acid in mg per g of protein): histidine 19; isoleucine 28; leucine 66; lysine 58; methionine + cysteine 25; phenylalanine + tyrosine 63; threonine 34; tryptophan 11; and valine 35. The amino acid score of each sample was taken as the value of the amino acid with the lowest ratio value among the 9 essential amino acids.
In vitro protein digestibility and in vitro protein digestibility corrected amino acid score
The in vitro protein digestibility (IVPD) of each sample was determined utilizing the pH-drop method (Tinus et al. 2012). A multi-enzyme solution was prepared fresh daily by mixing 31 mg of chymotrypsin (bovine pancreas ≥ 40 units per mg of protein), 16 mg of trypsin (porcine pancreas 13,000–20,000 BAEE units per mg of protein) and 13 mg of protease (Streptomyces griseus ≥ 15 units/mg solid) in 10 mL water. The enzyme solution was heated to 37 °C and adjusted to pH 8.0 using 0.1 M NaOH. The samples were prepared by dispersing 62.5 mg protein (corrected on a weight basis for protein content) in 10 mL of water, pre-heated to 37 °C. The solution was stirred for 1 h at 37 °C, and adjusted to pH 8.0 using 0.1 M NaOH. A 1 mL aliquot of the multi-enzyme cocktail was added to the protein solution at which point the pH of the protein solution was recorded every 30 s for 10 min. The in vitro protein digestibility (IVPD) was calculated using Eq. (1):
| 1 |
where ΔpH10 min is the change in pH from the initial pH of 8.0 to the pH at the end of 10 min. The in vitro protein digestibility corrected amino acid score (IV-PDCAAS) was calculated by multiplying the in vitro protein digestibility by the amino acid score.
Surface charge
The surface charge, as a function of pH (7.0–3.0), was determined by measuring the electrophoretic mobility (UE) of the protein using a Zetasizer Nano (Malvern Instruments, Westborough, USA). Protein solutions were made by dispersing the samples (0.05% protein, w/w) in water and stirring, using a magnetic stir plate (RO 5; IKA Works Inc., Wilmington, USA), overnight at room temperature. The electrophoretic mobility was then measured every 0.5 pH point over the pH range of 7.0–3.0 (pH adjustment using 0.1 M HCl or NaOH). The electrophoretic mobility is the measure of the velocity of a particle within an electric field, which can be related to the zeta potential (ζ) using the Henry equation (Eq. 2), where η is the dispersion viscosity, ε is the permittivity, and f(κα) is a function related to the ratio of particle radius (α) and the Debye length (κ). Using the Smoluchowski approximation f(κα) equaled 1.5. Measurements were performed in triplicate.
| 2 |
Protein solubility
Protein solubility (%) was determined by dispersing 0.2 g protein (based on weight protein content within each sample) in 19 mL water, adjusting to the desired pH (3.0, 5.0 or 7.0) using either 0.5 N HCl or NaOH and stirring (500 rpm) for 1 h at room temperature. Total solution weight was subsequently brought to 20.0 g with water. After centrifuging (VWR clinical centrifuge 200, VWR International, Mississauga, Canada) at 4180×g for 10 min at room temperature the protein content of the supernatant was determined using micro-Kjeldahl digestion and distillation units (model 6030000, micro-Kjeldahl digestor; and Rapid Distillation Glassware, Labconco, Kansas City, USA). Percent solubility was calculated by dividing the protein content in the supernatant by the protein content of the initial sample (×100).
Water hydration capacity and oil holding capacity
Water hydration capacity (WHC) and oil holding capacity (OHC) were determined by suspending 1.0 g of protein in 10.0 g of water/oil in a 50 mL screw capped centrifuge tube. Samples were vortexed for 10 s every 5 min for 30 min total then centrifuged (VWR clinical centrifuge 200, VWR International, Mississauga, Canada) for 15 min at 1000×g. After decanting the supernatant, the remaining pellet was weighed. The WHC and OHC were reported as the amount of water/oil absorbed per g of sample.
Foaming capacity and foam stability
Foaming properties for each protein sample were determined according to Liu et al. (2010), with slight modifications, by dispersing 2.00 g sample in 50 mL water. The solution pH was adjusted to 7.0 and then stirred overnight at room temperature. After readjusting to pH 7.0, 15 mL (initial liquid volume; VLi) of the solution was transferred into a narrow 400 mL glass beaker (inner diameter = 69 mm; height = 127 mm; as measured by a digital caliper) and foamed using an Omni Macro homogenizer (Omni International, Marietta, USA), equipped with a 20 mm saw tooth generating probe, for 5 min at speed 4 (~ 7200 rpm). After which in a 50 mL graduated cylinder the foam volume was measured at time zero (VF0) (immediately following homogenization) and after 30 min (VF30). Foaming capacity (FC) and foam stability (FS) were determined using Eqs. (3) and (4), respectively.
| 3 |
| 4 |
Statistical analysis
A one-way ANOVA with Tukey’s post hoc test was used to detect statistical differences between the different protein samples for proximate analysis, each protein fraction, each functional test, in vitro protein digestibility, and in vitro protein digestibility corrected amino acid score. All statistical analyses were performed with SigmaPlot version 14.0 (Systat Software, Inc., San Jose, USA).
Results and discussion
Proximate analysis
The composition of the four samples is reported on a dry basis (db) in Table 1. The cricket and mealworm powders had similar protein contents at 65.5 and 66.0% protein, respectively. The air classified faba bean protein concentrate contained 62.5% protein whereas yellow pea contained the least amount of protein (55.1%) of the four samples. The insect powders had much higher lipid contents (cricket 16.1%; mealworm 13.7%) than the pulse protein concentrates (faba bean 2.5%; yellow pea 2.1%). Hexane extraction, aqueous extraction, supercritical CO2 extraction, and ethanol extraction have all been used as methods to decrease the lipid content of insect flours (Zhao et al. 2016; Ndiritu et al. 2017; Purschke et al. 2018). Ash contents were similar among the four samples (4.3–5.5%). The compositions of the pulse protein concentrates were well within the ranges that have previously been reported for air classified pea and faba bean concentrates (Pelgrom et al. 2013; Martinez et al. 2016; Felix et al. 2018). The proximate composition of the cricket is similar to previously reported values. Zielińska et al. (2015) found G. sigillatus to contain 70.0% protein (d.b.), 18.23% lipid (d.b.) and 4.74% ash whereas Adebowale et al. (2005) reported large African cricket (Gryllidae sp.) to contain 65% crude protein (d.b.), 7–11% crude lipid (d.b.) depending on the sex, and 5% ash (d.b.). The proximate composition of mealworm larvae in this study is different than what has been reported in literature. For example, Purschke et al. (2018) found mealworm to have a crude lipid content of 22.7% (d.b.), and a protein content of 53.3%, however the protein value did not include chitin-bound nitrogen which has been reported to account for 6.8% of the total nitrogen content (Finke 2007). Like the aforementioned study, crude protein contents from 51 to 52% (d.b.) and lipid contents from 24 to 34% (d.b.) have been reported for mealworm larvae (Zhao et al. 2016; Yi et al. 2013; Bosch et al. 2014; Zielińska et al. 2015). However, ash content of the mealworm was similar to what has been reported by Bosch et al. (2014) at 3.9%, and Zhao et al. (2016) at 4.9%.
Table 1.
Proximate analysis and Osborne solubility protein fractions of commercial insect powders and pulse protein concentrates on a dry weight basis
| Source | Lipid (%) | Ash (%) | Protein (%) | Protein fractions (% of total protein) | |||
|---|---|---|---|---|---|---|---|
| Albumin | Globulin | Glutelin | Prolamin | ||||
| Cricket | 16.1 ± 0.1a | 4.3 ± 0.0a | 65.5 ± 0.5a | 31.5 ± 0.3a | 30.6 ± 0.3a | 13.7 ± 0.5a | 24.2 ± 0.6a |
| Mealworm | 13.7 ± 0.8b | 5.2 ± 0.1b | 66.0 ± 0.3a | 32.0 ± 0.1a | 31.2 ± 0.1b | 10.9 ± 0.5b | 25.8 ± 0.2a |
| Faba bean | 2.5 ± 0.5c | 5.3 ± 0.1b | 62.5 ± 0.6b | 45.4 ± 0.4b | 46.2 ± 0.9c | 4.3 ± 0.2c | 4.1 ± 0.2b |
| Yellow pea | 2.1 ± 0.3c | 5.5 ± 0.0c | 55.1 ± 1.2c | 44.2 ± 0.8b | 46.6 ± 0.7c | 6.4 ± 0.3d | 2.8 ± 0.2b |
Data represents the mean ± one standard deviation (n = 3). Different letters within a column indicate significantly different values (p < 0.05)
Variation within species is well documented; for example, Marono et al. (2015) found crude protein contents ranging from 51.8 to 59.0% for 6 different mealworm flours obtained from 3 different suppliers. Different proximate compositions within the same species is related to the diet, gut content at harvest, growing/farming environment, and crude protein determinations including chitin-bound nitrogen (Nowak et al. 2016; Finke 2007). For instance mealworms usually contain only small amounts of calcium but feeding them a high calcium diet can result in a calcium content that obtains a food labelling ‘source of’ calcium claim (Hunt et al. 2001; Nowak et al. 2016). Although not measured in this study crickets and mealworms both contain a significant amount of fibre (~ 4–13% d.b.), this includes chitin, an insoluble fibre with potential health benefits (FAO 2013; Stull et al. 2018; Finke 2002; Ndiritu et al. 2017; Purschke et al. 2018).
Osborne solubility fractions
The protein content of each fraction recovered from extraction based on the Osborne classification scheme is reported as percent of total protein recovered in Table 1. Both insect proteins contained similar amounts of albumin and globulin at 32% and 31%, respectively, of the total protein. The third most abundant fraction was prolamin representing 24.2 and 25.8% of the total protein in cricket and mealworm, respectively. Glutelin was the least abundant fraction in the cricket (13.7%) and mealworm (10.9%) proteins although significant amounts were still present. Ndiritu et al. (2017) reported a house cricket (Acheta domesticus) protein concentrate to be composed of over 40% globulin, with glutelin being the second most abundant fraction followed by albumin then prolamin; furthermore the authors found that protein extraction method had an impact on the fraction distribution. In comparison, the pulse proteins contained a higher proportion of albumin and globulin and lower proportion of glutelin and prolamin than the insect proteins with the largest difference being in the prolamin fraction. The insect proteins contained over 5 and 8 times the amount of prolamin as the faba bean and yellow pea, respectively.
Protein quality
The amino acid composition of the samples is reported in Table 2 on an “as is” basis. These values have been used in conjunction with the FAO/WHO (1991) reference pattern to calculate the essential amino acid scores which can be found in Table 3. Values less than 1 indicate the sample is deficient in that amino acid; the lowest value among the nine essential amino acids is the amino acid score. Both pulse proteins were first limiting in tryptophan with faba bean having a score of 0.69 and yellow pea a score of 0.70; cricket was also first limiting in tryptophan but had a higher score of 0.85 (p < 0.05). Mealworm had a similar score to the pulse proteins, with a value of 0.71, however was first limiting in lysine. The amino acid scores of the insect powders make them incomplete proteins but comparable to plant proteins. Due to studies utilizing different FAO/WHO scoring patterns, amino acid values reported in the literature have been compared to the FAO/WHO 1991 reference pattern utilized in the current study to compare values and overall protein quality. Amino acid profiles reported for both mealworm and cricket indicated that neither insect met the requirements for leucine, isoleucine, lysine, and total aromatic amino acids (phenylalanine + tyrosine) (Zielińska et al. 2015), whereas findings from Adebowale et al. (2005) for large African crickets (Gryllidae sp.) indicated that the female crickets were a complete protein whereas the male crickets were slightly deficient in tryptophan containing 10.5 mg/g protein compared to the 11 mg/g protein required by the 1991 FAO/WHO reference pattern. Comparing the amino acid profile of mealworm reported by Zhao et al. (2016), with the exception of tryptophan which was not measured, to the FAO/WHO (1991) reference pattern shows that the sulphur amino acids combined (methionine + cysteine) were the limiting amino acid with a score of 0.85, similar to the value of 0.83 found in this study for the sulphur amino acids. Zielińska et al. (2015) also reported mealworm to be deficient in total sulphur amino acids in addition to threonine. In contrast the values reported by Yi et al. (2013) for mealworm show that lysine was the only amino acid below the required FAO/WHO (1991) value whereas in the current study mealworm was also deficient in tryptophan, sulphur amino acids and threonine. Gryllodes sigillatus is not the only cricket species that is an incomplete protein; house cricket (A. domesticus) has been reported to be deficient in tryptophan and lysine (Yi et al. 2013) based on the FAO/WHO (1991) reference pattern.
Table 2.
Amino acid composition (g per 100 g of flour, as is basis) of commercial insect powders and pulse protein concentrates
| Source | ASP | THR | SER | GLU | PRO | GLY | ALA | CYS | VAL | MET | ILE | LEU | TYR | PHE | HIS | LYS | ARG | TRP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cricket | 6.17 | 2.17 | 4.77 | 7.92 | 4.22 | 3.71 | 5.20 | 0.97 | 3.67 | 1.50 | 2.84 | 4.69 | 3.07 | 2.36 | 1.84 | 3.50 | 4.62 | 0.60 |
| Mealworm | 4.99 | 1.94 | 4.26 | 7.27 | 4.14 | 3.35 | 5.10 | 0.60 | 3.45 | 0.72 | 2.54 | 4.43 | 3.42 | 1.95 | 2.68 | 2.62 | 3.08 | 0.57 |
| Faba bean | 11.43 | 1.48 | 3.35 | 11.54 | 2.28 | 2.14 | 1.92 | 0.94 | 2.29 | 0.41 | 2.20 | 4.07 | 1.67 | 2.32 | 1.07 | 3.19 | 5.33 | 0.46 |
| Yellow pea | 6.16 | 1.49 | 2.99 | 9.26 | 2.27 | 2.03 | 2.14 | 0.88 | 2.28 | 0.61 | 2.12 | 3.78 | 1.69 | 2.51 | 1.43 | 3.75 | 4.48 | 0.41 |
ASP aspartate, THR threonine, SER serine, GLU glutamate, PRO proline, GLY glycine, ALA alanine, CYS cysteine, VAL valine, MET methionine, ILE isoleucine, LEU leucine, TYR tyrosine, PHE phenylalanine, HIS histidine, LYS lysine, ARG arginine, TRP tryptophan
Table 3.
Essential amino acid scores, in vitro protein digestibility values, and in vitro protein digestibility corrected amino acid scores of commercial insect powders and pulse protein concentrates
| Source | THR | VAL | MET + CYS | ILE | LEU | PHE + TYR | HIS | LYS | TRP | Amino Acid Score | In vitro protein digestibility (%)1 | IV-PDCAAS1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cricket | 0.99 | 1.63 | 1.53 | 1.57 | 1.10 | 1.34 | 1.50 | 0.94 | 0.85 | 0.85 | 76.2 ± 0.1a | 0.65 ± 0.00a |
| Mealworm | 0.90 | 1.55 | 0.83 | 1.43 | 1.06 | 1.34 | 2.22 | 0.71 | 0.82 | 0.71 | 75.7 ± 0.2a | 0.54 ± 0.01b |
| Faba bean | 0.72 | 1.08 | 0.89 | 1.29 | 1.01 | 1.04 | 0.93 | 0.90 | 0.69 | 0.69 | 80.9 ± 0.2b | 0.56 ± 0.00c |
| Yellow pea | 0.82 | 1.22 | 1.11 | 1.41 | 1.07 | 1.24 | 1.40 | 1.21 | 0.70 | 0.70 | 83.7 ± 0.2c | 0.59 ± 0.00d |
1Data represents the mean ± one standard deviation (n = 3). Different letters within a column indicate significantly different values (p < .05)
Bolded values reflect the first limiting amino acid
THR threonine, VAL valine, MET methionine, CYS cysteine, ILE isoleucine, LEU leucine, PHE phenylalanine, TYR tyrosine, HIS histidine, LYS lysine, TRP tryptophan, IV-PDCAAS, in vitro protein digestibility corrected amino acid score
The in vitro protein digestibility (IVPD) is presented in Table 3. The insect powders had similar digestibility values (approx. 76%), whereas the pulse protein concentrates had significantly (p < 0.05) higher digestibility values at 80.9% for faba bean and 83.7% yellow pea. However, this did not translate into higher in vitro protein digestibility corrected amino acid scores (IV-PDCAAS), the product of the amino acid score and the IVPD, as the cricket protein had the highest IV-PDCAAS (0.65) of the four samples, indicating it had the best protein quality. This was followed by the yellow pea and the faba bean protein concentrates with IV-PDCAAS of 0.59 and 0.56, respectively. The mealworm had the lowest IV-PDCAAS (0.54) and was therefore the lowest quality protein of the four samples. In vitro protein digestibility values of 90.7 and 94.7% have been reported for male and female large African crickets (Gryllidae sp.), respectively (Adebowale et al. 2005). For mealworm larvae, Bosch et al. (2014) reported an IVPD of 91.3% whereas Marono et al. (2015) obtained a value of approx. 66%. In vitro crude protein digestibility of insect meals (mealworms and black soldier flies) has been negatively correlated with chitin (Marono et al. 2015).
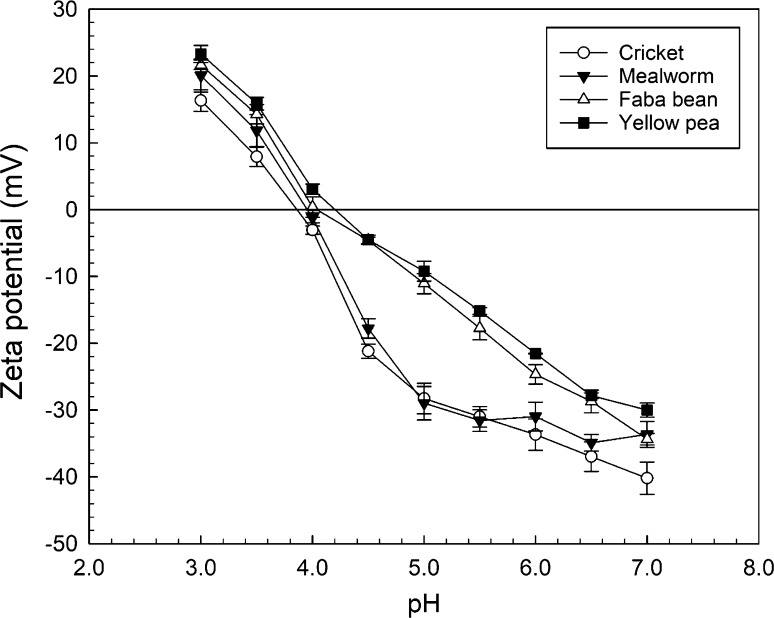
Surface charge
Figure 1 shows the surface charge (zeta potential, mV) of the commercial insect powders and pulse protein concentrates as a function of pH (7.0–3.0). The isoelectric point (0 mV) of each protein ingredient was as follows: cricket, pH 3.85; mealworm, pH 3.95; faba bean, pH 4.05; and yellow pea, pH 4.20. At pH 7.0 the cricket protein was the most negatively charged (− 40.2 mV), followed by mealworm and faba bean which had similar charges (approx. − 34.0 mV) and then yellow pea (− 30.0 mV). As the pH was lowered the pulse proteins followed a similar trend as each other becoming less negatively charged in a linear fashion from approx. − 28.0 to − 4.5 mV from pH 6.5–4.5; after reaching their isoelectric points the proteins assumed a positive charge of > 20.0 mV at pH 3.0. The charge on the mealworm protein fluctuated < 5 mV over the pH range 7.0–5.0 before becoming less negatively charged at pH 4.5 (− 17.8 mV) and then sharply increasing to − 1.2 mV at pH 4.0. After reaching its pI just below pH 4.0 the protein assumed a net positive charge of 11.8 and 20.1 mV at pH 3.5 and 3.0, respectively. The cricket protein followed a similar trend to the mealworm protein becoming sharply less charged from pH 4.5 to 4.0 (− 21.2 to − 3.1 mV). The cricket protein had the lowest charge of the four proteins at pH 3.0 (16.3 mV).
Fig. 1.
Surface charge (zeta potential, mV) as a function of pH for commercial insect powders and pulse protein concentrates. Data represents the mean ± one standard deviation (n = 3)
Functionality
Solubility
The solubility of the commercial protein products was determined at pH 3.0, 5.0, and 7.0 (Table 4). The pH did not affect the solubility of the insect proteins whereas the pulse proteins had much different solubility values at each pH. At pH 3.0 the cricket protein (29.2%) was more soluble than faba bean (22.3%) but less soluble than the yellow pea (36.0%) which had the highest solubility at that pH (p < 0.05), whereas the mealworm protein performed slightly better than only the faba bean protein, with a solubility of 24.6%. The solubility’s of the pulse proteins declined at pH 5.0, near their isoelectric points, dropping to 14.0 and 16.8% for the faba bean and yellow pea, respectively. In contrast, the cricket protein maintained the same level of solubility at pH 5.0 as at pH 3.0 whereas the mealworm protein had a small decrease in solubility at pH 5.0 (22.3%). The higher solubility than the pulse proteins at pH 5.0 may be related to the higher surface charge of insect proteins at this pH (Fig. 1). At pH 7.0 the insect proteins remained consistent in their solubility values whereas the faba bean and yellow pea proteins experienced a large increase in solubility reaching 80.9 and 80.0% soluble, respectively. The overall low solubility of the insect proteins may be related to the type of protein present in the powders (i.e. less albumin and globulin, and more glutelin and prolamin than the pulse proteins), the insect proteins have higher proportion of non-polar amino acids as compared to the pulse proteins, as well as the heating of the insects during processing is hypothesized to result in protein denaturation exposing hydrophobic groups leading to protein aggregation. Zhao et al. (2016) reported the solubility of defatted mealworm protein to be the lowest at pH 4 and 5 (< 10%), increasing slightly at pH 3 (approx. 16%), becoming almost 40% soluble at pH 7.0, while achieving the greatest solubility at pH 9.0 reaching over 70% soluble. Hall et al. (2017) measured the protein solubility of cricket at pH 3.0, 7.0, 8.0, and 10.0 and reported the highest solubility reported at pH 10.0 (< 30%) and lowest at pH 3.0 (< 10%); however enzymatic hydrolysis greatly improved the protein solubility at all pHs measured. Adebowale et al. (2005) reported the solubility of large African cricket (Gryllidae sp.) to be 19% at both pH 3.0 and 5.0 with an increase in solubility to 29% at pH 7.0. Zielińska et al. (2018) studied the solubility of mealworm and cricket flours from pH 2.0 to 11.0 with minimum values observed at pH 5.0 with values of 3% for mealworm and 4% for cricket. The authors reported similar solubility values as the current study at pH 7.0 but found higher solubility at pH 3.0 (mealworm 52%; cricket 65%).
Table 4.
Functional properties of commercial insect powders and pulse protein concentrates
| Source | Solubility (%) | Water hydration capacity (g/g) | Oil holding capacity (g/g) | Foaming capacity1 (%) | Foam stability1 (%) | ||
|---|---|---|---|---|---|---|---|
| pH 3.0 | pH 5.0 | pH 7.0 | |||||
| Cricket | 29.2 ± 0.8a | 29.6 ± 1.7a | 28.2 ± 0.3a | 1.76 ± 0.00a | 1.42 ± 0.05a | 82 ± 4a | 86 ± 5a |
| Mealworm | 24.6 ± 0.2b | 22.3 ± 0.5b | 23.2 ± 0.2b | 1.62 ± 0.01b | 1.58 ± 0.05b | Non-foaming | Non-foaming |
| Faba bean | 22.3 ± 0.3c | 14.0 ± 0.1c | 80.9 ± 1.2c | 0.95 ± 0.03c | 1.48 ± 0.04ab | 149 ± 4b | 62 ± 3b |
| Yellow pea | 36.0 ± 0.8d | 16.8 ± 0.4d | 80.0 ± 1.0c | 1.60 ± 0.02b | 1.31 ± 0.01c | 149 ± 4b | 49 ± 1c |
1Determined at pH 7
Data represents the mean ± one standard deviation (n = 3). Different letters within a column indicate significantly different values (p < .05)
Water hydration capacity and oil holding capacity
The functional properties of the commercial insect and pulse protein products are presented in Table 4. The cricket protein had the highest (p < 0.05) water hydration capacity (WHC) at 1.76 g/g whereas the mealworm and yellow pea proteins had similar and slightly lower WHC at 1.62 and 1.60 g/g, respectively. The faba bean protein had the lowest (p < 0.05) WHC (0.95 g/g) of the four samples. There were only small differences in the oil holding capacity (OHC) values of the protein products, with mealworm having the highest OHC (1.58 g/g) however this was not significantly (p > 0.05) different than faba bean protein which then had a similar (p > 0.05) value as the cricket protein, at 1.48 and 1.42 g/g, respectively. The yellow pea had an OHC of 1.31 g/g which was the lowest (p < 0.05) of all the samples. Water and oil holding abilities are important in many food applications for mouthfeel, texture, palatability, ingredient binding, etc. (Barbut 1996). Zielińska et al. (2018) reported similar water and oil holding capacities for mealworm flour but higher values for cricket flour. Zhao et al. (2016) reported water and oil absorption capacities of 1.87 g/g and 2.33 g/g, respectively, for a mealworm protein extract whereas Adebowale et al. (2005) reported the water and oil absorption capacities of large African cricket (Gryllidae sp.) to be 2.38 and 2.02 g/g, respectively. Protein extraction from insect meal/flour can be used to increase WHC and OHC as Ndiritu et al. (2017) found house cricket (A. domesticus) protein extracts to range from 2.0 to 2.7 g/g for WHC and 3.37–3.53 g/g for OHC depending on extraction method, and Zielińska et al. (2018) reported mealworm and cricket protein extracts to have higher WHC and OHC than their respective flours.
Foaming capacity and foam stability
The foaming properties of the commercial protein products at pH 7.0 are reported in Table 4. The mealworm powder did not produce any foam under the conditions utilized and was therefore classified as non-foaming. The cricket powder had a foaming capacity (FC) of 82% which was significantly (p < 0.05) lower than the faba bean and yellow pea protein concentrates which both had FC values of 149%. The much lower solubility at pH 7.0 for the cricket protein, as compared to the pulse proteins, is hypothesized to have inhibited the protein migration to the air water interface which is needed for foam formation (Wilde and Clark 1996). The high lipid content may have also prevented foaming. In contrast to the FC, the cricket protein had the best foam stability (FS) value (86%) followed by the faba bean protein (62%) and then yellow pea protein (49%). Foam stabilization requires a higher amount of hydrophobicity or insolubility in order for the viscoelastic film to remain at the air–water interface and prevent foam breakdown (Wilde and Clark 1996). Hall et al. (2017) reported cricket to have a FC of approx. 90% and a 60-min FS of approx. 80% for a 3% protein solution at pH 6.8, whereas Adebowale et al. (2005) reported that large African cricket (Gryllidae sp.) had a very low FC of 6% and a 2-h FS of 3%. These inconsistencies may be the result of method differences or high variation within species. Zielińska et al. (2018) reported cricket to have a higher FC (41%) and FS (35%) than mealworm (30% FC; 25% FS) which they hypothesized to be due to higher levels of carbohydrate (sugars) present in the mealworm. Similar to the mealworm powder in this study, Nditritu et al. (2017) reported that extracted house cricket (A. domesticus) protein concentrates were not sufficient for foaming applications due to their very poor foaming capacity and stability.
In general the pea and faba bean protein concentrates had comparable physicochemical properties as other commercial plant protein concentrates as reported in Martinez et al. (2016).
Conclusion
This study compared the physicochemical properties and protein quality of cricket (Gryllodes sigillatus) and mealworm (Tenebrio molitor) powders with those of commercial pulse protein concentrates from yellow pea and faba bean. The insect proteins had comparable WHC and OHC to that of the pulses with the exception of faba bean having an inferior WHC. The pulse proteins produced much more foam than the cricket protein however the cricket protein foam had a higher 30-min stability. The mealworm protein was non-foaming and is therefore not suitable for applications requiring foam. The lipid contents of the insect powders were much higher than the pulse proteins which may have negatively influenced some of the functional properties of the insect proteins. The pH did not have a large effect on insect protein solubility whereas the pulse proteins had decreased solubility at pH 5.0 and high solubility at pH 7.0. The cricket protein had the best protein quality of the four proteins having the highest in vitro protein digestibility corrected amino acid score, whereas the mealworm protein had the lowest. The isoelectric points of all samples were within 0.5 pH point, from 3.85 for cricket to 4.20 for yellow pea and mealworm. Overall the insect powders are well suited for use as novel protein sources with more research needed into their applicability as ingredients in specific food products where high solubility is not a requirement such as extruded snack products, binders in meat products, nutrition/sports bars, and bakery products.
Acknowledgements
Financial support for this research was provided through the Saskatchewan Ministry of Agriculture Strategic Research Chair Program in Protein Quality and Utilization. Special thanks to Kelsey Waelchli and Renbo Xu who provided laboratory support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adebowale YA, Adebowale KO, Oguntokun MO. Evaluation of nutritive properties of the large African cricket (Gryllidae sp) Pak J Sci Ind Res. 2005;48:274–278. [Google Scholar]
- AOAC International . Official methods of analysis of AOAC International. Gaithersburg: AOAC International; 2005. [Google Scholar]
- Azzollini D, Derossi A, Fogliano V, Lakemond CMM, Severini C. Effects of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov Food Sci Emerg Technol. 2018;45:344–353. doi: 10.1016/j.ifset.2017.11.017. [DOI] [Google Scholar]
- Barbut S. Determining water and fat holding. In: Hall GM, editor. Methods of testing protein functionality. London: Chapman & Hall; 1996. pp. 186–225. [Google Scholar]
- Bosch G, Zhang S, Oonincx D, Hendriks W. Protein quality of insects as potential ingredients for dog and cat foods. J Nutr Sci. 2014;3(e29):1–4. doi: 10.1017/jns.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuj-Laines R, Hernández-Santos B, Reyes-Jaquez D, Delgado-Licon E, Juárez-Barrientos JM, Rodríguez-Miranda J. Physicochemical properties of ready-to-eat extruded nixtamalized maize-based snacks enriched with grasshopper. Int J Food Sci Technol. 2018;53:1889–1895. doi: 10.1111/ijfs.13774. [DOI] [Google Scholar]
- FAO (2013) Edible insects—future prospects for food and feed security. FAO forestry paper, vol 171. Food and Agriculture Organization of the United Nations, Rome
- FAO/WHO (1991) Protein quality evaluation: Report of the joint FAO/WHO expert consultation. Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO), Rome
- Felix M, Lopez-Osorio A, Romero A, Guerrero A. Faba bean protein flour obtained by densification: a sustainable method to develop protein concentrates with food applications. LWT. 2018;93:563–569. doi: 10.1016/j.lwt.2018.03.078. [DOI] [Google Scholar]
- Finke MD. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002;21:69–285. doi: 10.1002/zoo.10031. [DOI] [Google Scholar]
- Finke MD. Estimate of chitin in raw whole insects. Zoo Biol. 2007;26:105–115. doi: 10.1002/zoo.20123. [DOI] [PubMed] [Google Scholar]
- González CM, Garzón R, Rosell CM. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innov Food Sci Emerg Technol. 2018;2:3. [Google Scholar]
- Hall FG, Jones OG, O’Haire ME, Liceaga AM. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017;224:414–422. doi: 10.1016/j.foodchem.2016.11.138. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Shi J, Giusto A, Siegrist M. The psychology of eating insects: a cross-cultural comparison between Germany and China. Food Qual Prefer. 2015;44:148–156. doi: 10.1016/j.foodqual.2015.04.013. [DOI] [Google Scholar]
- Henchion M, Hayes M, Mullen AM, Fenelon M, Tiwari B. Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods. 2017;6:53. doi: 10.3390/foods6070053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt AS, Ward AM, Ferguson G (2001) Effects of a high calcium diet on gut loading in varying ages of crickets (Acheta domestica) and mealworms (Tenebrio molitor). In: Edwards MS, Lisi KJ, Schlegel ML, Bray R (eds) Proceedings of the 4th conference on zoo and wildlife nutrition, Lake Buena Vista, pp 94–99
- Kim HW, Setyabrata D, Lee YJ, Jones OG, Kim YHB. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov Food Sci Emerg Technol. 2016;38:116–123. doi: 10.1016/j.ifset.2016.09.023. [DOI] [Google Scholar]
- Landry J, Delhaye S. Determination of tryptophan in feedstuffs: comparison of sodium hydroxide and barium hydroxide as hydrolysis agents. Food Chem. 1994;49:95–97. doi: 10.1016/0308-8146(94)90238-0. [DOI] [Google Scholar]
- Liu S, Elmer C, Low NH, Nickerson MT. Effect of pH on the functional behaviour of pea protein isolate-gum Arabic complexes. Food Res Int. 2010;43:489–495. doi: 10.1016/j.foodres.2009.07.022. [DOI] [Google Scholar]
- Marono S, Piccolo G, Loponte R, Di Meo C, Attia YA, Nizza A, Bovera F. In vitro crude protein digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Ital J Anim Sci. 2015;14:338–3434. doi: 10.4081/ijas.2015.3889. [DOI] [Google Scholar]
- Martinez M, Stone AK, Yovchev AG, Peter R, Vandenberg A, Nickerson MT. Effect of genotype and environment on the surface characteristics and functionality of air-classified faba bean protein concentrates. Eur Food Res Technol. 2016;242:1903–1911. doi: 10.1007/s00217-016-2690-4. [DOI] [Google Scholar]
- Nakagaki BJ, Defoliart GR. Comparison of diets for mass-rearing Acheta domesticus (Orthoptera: Gryllidae) as a novelty food, and comparison of food conversion efficiency with values reported for livestock. J Econ Entomol. 1991;84:891–896. doi: 10.1093/jee/84.3.891. [DOI] [Google Scholar]
- Ndiritu AK, Kinyuru JN, Kenji GM, Gichuhi PN. Extraction technique influences the physico-chemical characteristics and functional properties of edible crickets (Acheta domesticus) protein concentrate. Food Measure. 2017;11:2013–2021. doi: 10.1007/s11694-017-9584-4. [DOI] [Google Scholar]
- Nowak V, Persijn D, Rittenschober D, Charrondiere UR. Review of food composition data for edible insects. Food Chem. 2016;193:39–46. doi: 10.1016/j.foodchem.2014.10.114. [DOI] [PubMed] [Google Scholar]
- Oonincx DGAB, van Itterbeeck J, Heetkamp MJW, van den Brand H, van Loon JJA, van Huis A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE. 2010;5:e14445. doi: 10.1371/journal.pone.0014445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgrom PJM, Vissers AM, Boom RM, Schutyser MAI. Dry fractionation for production of functional pea protein concentrates. Food Res Int. 2013;53:232–239. doi: 10.1016/j.foodres.2013.05.004. [DOI] [Google Scholar]
- Purschke B, Brüggen H, Scheibelberger R, Jäger H. Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.) Eur Food Res Technol. 2018;244:269–280. doi: 10.1007/s00217-017-2953-8. [DOI] [Google Scholar]
- Schösler H, de Boer J, Boersema JJ. Can we cut out the meat of the dish? Constructing consumer-oriented pathways towards meat substitution. Appetite. 2012;58:39–47. doi: 10.1016/j.appet.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Stull VJ, Finer E, Bergmans RS, Febvre HP, Longhurst C, Manter DK, Patz JA, Weir TL. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci Rep. 2018;8:10762. doi: 10.1038/s41598-018-29032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinus T, Damour M, van Riel V, Sopade PA. Particle size-starch–protein digestibility relationships in cowpea (Vigna unguiculata) J Food Eng. 2012;113:254–264. doi: 10.1016/j.jfoodeng.2012.05.041. [DOI] [Google Scholar]
- Verbeke W. Profiling consumers who are ready to adopt insects as a meat substitute in a Western society. Food Qual Prefer. 2015;39:147–155. doi: 10.1016/j.foodqual.2014.07.008. [DOI] [Google Scholar]
- White DA, Hart RJ, Fry JC. An evaluation of the waters pico-tag system for the amino-acid analysis of food materials. J Anal Methods Chem. 1986;8:170–177. doi: 10.1155/S1463924686000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde PJ, Clark DC. Foam formation and stability. In: Hall GM, editor. Methods of testing protein functionality. London: Chapman & Hall; 1996. pp. 110–152. [Google Scholar]
- Yi L, Lakemond CMM, Sagis LMC, Eisner-Schadler V, van Huis A, van Boekel MAJS. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013;141:3341–3348. doi: 10.1016/j.foodchem.2013.05.115. [DOI] [PubMed] [Google Scholar]
- Zhao X, Vazquez-Gutierrez JL, Johansson DP, Landberg R, Langton M. Yellow mealworm protein for food purposes— extraction and functional properties. PLoS ONE. 2016;11:1–17. doi: 10.1371/journal.pone.0147791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska E, Baraniak B, Karaś M, Rybczyńska K, Jakubczyk A. Selected species of edible insects as a source of nutrient composition. Food Res Int. 2015;77:460–466. doi: 10.1016/j.foodres.2015.09.008. [DOI] [Google Scholar]
- Zielińska E, Karaś M, Baraniak B. Comparison of functional properties of edible insects and protein preparations thereof. LWT—Food Sci Technol. 2018;91:168–174. [Google Scholar]