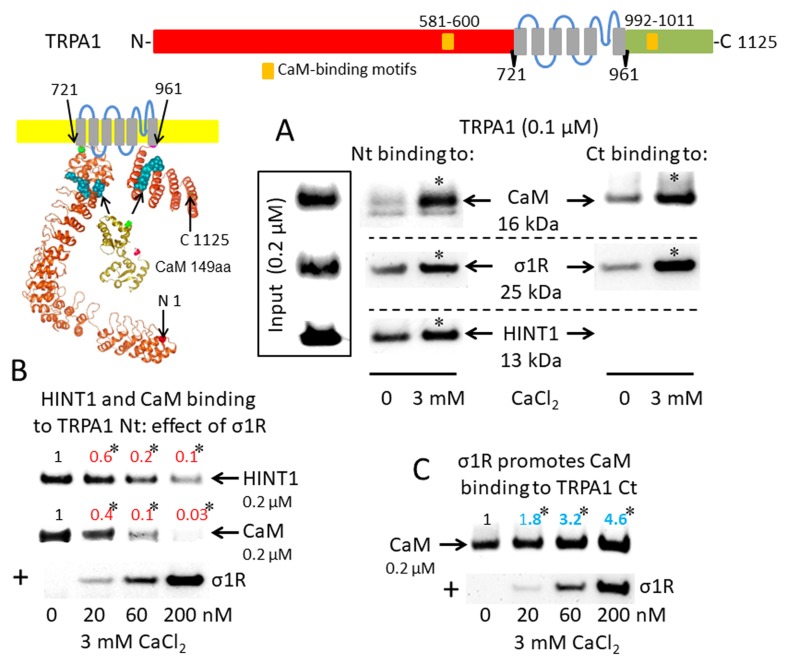
Figure 1.
Sigma 1 receptor (σ1R), histidine triad nucleotide-binding protein 1 (HINT1), and calmodulin (CaM) binding to the transient receptor potential ankyrin member 1 (TRPA1) calcium channel. The TRP structural models shown were predicted by Novafold (DNASTAR Inc., Madison, WI, USA). Linear model: the N- and C-terminal cytosolic sequences are red and green, respectively, and the six transmembrane domains are in gray. Ribbon model: The 3D structure of N- and C-terminal sequences is shown; the CaM-binding motifs are indicated by blue spheres. (A) The in vitro interactions of the σ1R, CaM, and HINT1 with TRPA1 were evaluated in co-precipitation assays. Recombinant N- and C-terminal regions of TRPA1 (100 nM) were co-incubated in the presence and absence of 3 mM CaCl2, with the input of 200 nM CaM, σ1R, and HINT1. The TRPA1 N-terminus (aa 1–721) or the TRPA1 C-terminus (aa 961–1125) were immobilized by covalent attachment to NHS-activated Sepharose. Prey proteins alone did not bind to the blocked NHS-Sepharose (negative control). (B and C) Competition assays between the σ1R and CaM or HINT1 for binding to the N- and C-terminal regions of TRPA1. After incubation in 3 mM CaCl2, the TRPA1-bound proteins were detached and resolved by SDS-PAGE chromatography, and analyzed in Western blots. The assays were repeated at least twice, producing comparable results. For the interactions with increased concentrations of the σ1R (up to 200 nM), the data are shown relative to that obtained in the absence of the σ1R, with the control group arbitrary assigned a value of 1. *Significant differences with respect to the control group, ANOVA and Dunnett multiple comparisons vs. control group, p < 0.05. Representative blots are shown.