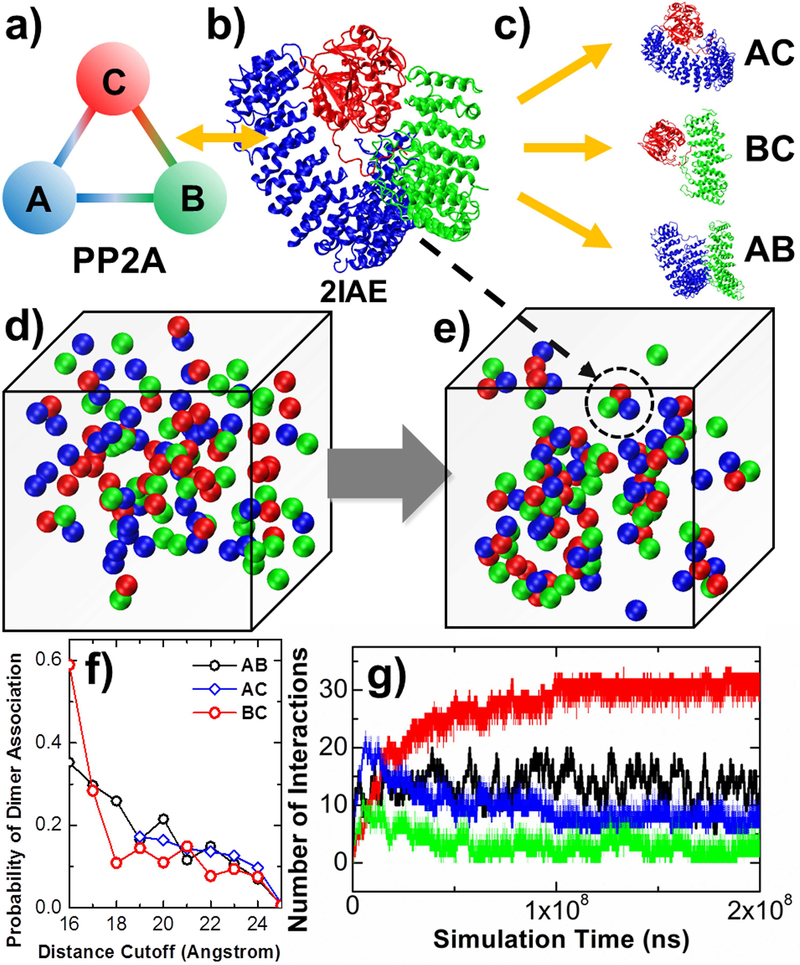
Figure 5:
We studied the assembling kinetics of a heterotrimeric holoenzyme, protein phosphatase 2A (PP2A) (a). The atomic coordinates of the entire protein complex are taken from the PDB id 2IAE (b). Residue-based simulations were first performed to three systems to test the binding between all pairs of subunits in the complex (c). In RB simulations, there are 120 rigid bodies in the system. Each rigid body belongs to one of the three types of subunits, as indicated in red, green and blue (d). Hetero-trimers that share the same quaternary arrangement as the crystal structure are found at the end of the simulation (e), one of which is highlighted in the dashed circle. The association probabilities between three pairs of subunits calculated from residue-based simulations are plotted in (f), under different values of distance cutoff. Finally, the RB simulation results are plotted in (g), with the number of trimeric complexes (red curve); the number of A-C dimers (blue curve); the number of B-C dimers (green curve) formed along simulation; and the number of B-C dimers (black curve) formed in a control simulation in which only rigid bodies of subunit B and C were included in the system.