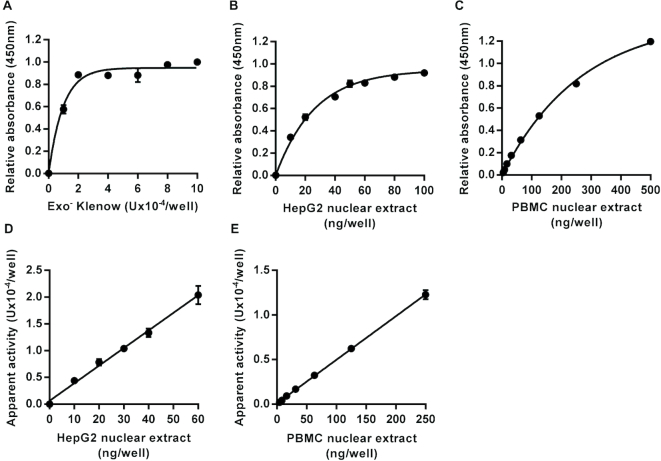
Figure 3.
Assay for DNA polymerase repair activity. Incubation of an immobilized hairpin loop oligonucleotide substrate containing a single nucleotide gap on one strand within the double-stranded region with exonuclease minus Klenow fragment of Escherichia coli DNA polymerase I, in the presence of dNTPs, ATP and excess T4 DNA ligase, led to an increase in intact substrate retained in the microplate wells following neutral denaturation that was dependent on the concentration of the polymerase (A). Incubation with HepG2 nuclear extract (B) or peripheral blood mononuclear cell pooled nuclear extract (C) also caused concentration-dependent increases in the amount of intact substrate retained in the wells. The apparent DNA polymerase activity was directly proportional to the concentration of nuclear extract over the range 0–0.06 μg/well for the HepG2 nuclear extract (r2 = 0.98) (D) and over the range 0–0.25 μg/well for the peripheral blood mononuclear cell nuclear extract (r2 = 0.99) (E). Data shown represent the mean ± SD of triplicate technical replicates.