Key Points
Question
For patients with head and neck squamous cell carcinoma, do positive margin (PM) rates vary according to treatment facility case volume, and are patients referred for chemoradiation therapy if they have PM?
Findings
For this retrospective cohort study, the National Cancer Database was used to identify 28 840 patients with an overall PM rate of 17.6%. In both univariable and multivariable analyses, higher facility volume was associated with a lower PM rate; however, no association was found between facility volume and likelihood of receiving adjuvant chemoradiation therapy in the setting of PM.
Meaning
Facility volume appears to be associated with patient likelihood of receiving a PM resection.
This retrospective cohort study uses data from the National Cancer Database to determine if there is an association between surgical treatment facility volume and positive margin rate in patients with head and neck squamous cell carcinoma.
Abstract
Importance
The achievement of complete tumor resection with tumor-free margins is one of the main principles of oncologic surgery for head and neck squamous cell carcinoma (HNSCC). The negative prognostic influence of a positive margin (PM) across all head and neck subsites has been well established. National guidelines recommend the use of adjuvant chemoradiation therapy (CRT) in the setting of PM.
Objective
To determine the incidence of PM in HNSCC across multiple subsites, as well as the factors associated with its occurrence.
Design, Setting, and Participants
This retrospective cohort study used the National Cancer Database to identify patients diagnosed with HNSCC between 2010 and 2014 and who underwent surgical resection (n = 28 840).
Main Outcomes and Measures
Predictors of PM rate and likelihood to receive adjuvant CRT.
Results
Among the 28 840 patients included in this study, 19 727 (68.4 %) were men, and the average age was 62.4 years (range, 40 to ≥90 years). In univariable analysis, a lower PM rate was associated with higher facility volume (26.3% for the lowest volume quartile, 16.5% for the middle 2 quartiles, and 10.8% for the highest volume quartile) and treatment at academic vs nonacademic facilities (14.0% vs 22.7%). In multivariate analysis, those treated at higher-volume facilities remained significantly less likely to have PM (adjusted odds ratio, 0.85; 95% CI, 0.83-0.88). The trend of decreasing PM rate with increasing facility volume was observed in both academic (aOR, 0.88 per 10-case volume increase [95% CI, 0.85-0.91]) and nonacademic (aOR, 0.73 per 10-case volume increase [95% CI, 0.68-0.80]) facilities. There was no association between facility volume and patient likelihood of receiving adjuvant CRT in the setting of PM (compared with CCPs: aOR, 0.98 per 10-case volume increase [95% CI, 0.84-1.14] for CCCPs; and aOR, 1.24 [95% CI, 0.99-1.55] for INCPs).
Conclusions and Relevance
These findings suggest that high-volume facilities are associated with lower rates of PM in the surgical treatment of HNSCC in both academic and nonacademic settings. Facility volume for head and neck oncologic surgeries may be considered a benchmark for quality of care.
Introduction
One of the main principles of head and neck oncologic surgery is the achievement of complete tumor resection and tumor-free margins.1 The negative prognostic effect of a positive margin (PM) in head and neck squamous cell carcinoma (HNSCC) has been well established.2,3,4,5,6,7 Although there are a number of pathologic features that place patients at high risk for local recurrence (eg, involved lymph nodes, extracapsular extension, perineural and lymphovascular invasion), PM is the only one that has potential for intraoperative modification. If PM is identified after surgical resection of HNSCC, National Comprehensive Cancer Network (NCCN) guidelines recommend the use of adjuvant chemoradiation therapy (CRT).
There are growing data in head and neck cancer literature on the association between outcomes and surgery-related factors. Large population-based studies have demonstrated positive associations between survival rates and surgical case volume, of both the surgeon and the treating hospital.8,9 Furthermore, the influence of the type of treating hospital has also been studied, with academic facilities producing better outcomes than nonacademic facilities among patients treated with adjuvant radiation therapy.10,11 Regarding PM and surgery-related factors, a high rate of PM has been associated with treatment at nonacademic facilities and institutions with low oral cancer case volume, but these studies are limited in scope with respect to head and neck subsites and staging.12,13
Using a contemporary data set, our aim was to determine the incidence of PM in HNSCC of all stages across multiple subsites and the factors associated with PM occurrence. We hypothesized that margin status is associated with treatment facility characteristics and that the prescription of adjuvant CRT is different across facility types.
Methods
Data Source
Data for this study came from the National Cancer Database (NCDB), a joint project of the American Cancer Society and the American College of Surgeons Commission on Cancer (CoC). The database captures more than 80% of newly diagnosed head and neck cancers from approximately 1500 CoC-accredited facilities around the United States.14 The NorthShore University HealthSystem Institutional Review Board granted this study exempt status, and patient informed consent was waived because the study was retrospective and data from the NCDB were deidentified.
Study Cohort
We identified patients diagnosed with invasive HNSCC from 2010 through 2014 who were then treated with definitive surgery. The cohort was limited to patients with clinical stage I through IVB tumors (excluding those with clinical T4b disease) of the oral cavity, oropharynx, larynx, and hypopharynx, based on the American Joint Committee on Cancer 7th edition.15 Those with grade 4 tumors, other invasive malignancies, and unknown or indeterminate margin status; who underwent local tumor destruction or biopsy; and who received care at facilities other than the diagnosing facility were excluded. We restricted the cohort to patients who received surgery at the facility that reported their cases to the NCDB. Finally, those younger than 40 years were excluded, because data on the type of treating facility (eg, academic, community) is unavailable in the NCDB for those younger than 40 years. We identified approximately 2900 patients (10.2% of the entire cohort) with TX listed for their pathologic T category. We ran a sensitivity analysis excluding this TX cohort and found that it did not affect the results.
Statistical Analysis
Unadjusted risk ratios for PM, with corresponding 95% CIs, were computed for demographic, clinical, and facility factors. Demographic factors included age, sex, race/ethnicity, insurance, and socioeconomic status (from an aggregate of county-level median income and education). Clinical factors included the Charlson-Deyo comorbidity score,16 primary site, tumor grade, clinical grouped TNM stage, pathologic T category, and pathologic N category. Facility characteristics included the facility mean annual surgical volume (grouped into <25th, 25th-75th, and >75th percentiles), facility location, and facility type (ie, academic, nonacademic). Nonacademic facilities were characterized by their lack of residency training program requirements and were categorized according to their annual caseloads. Nonacademic facilities included community cancer programs (CCPs) (100-500 new cases per year), comprehensive community cancer programs (CCCPs) (>500 new cases per year), and integrated network cancer programs (INCPs) (organization with multiple facilities related to cancer care, 1 of which is a CoC-accredited hospital; no minimum caseload). Surgical margins were coded into the NCDB as negative, positive (divided into macroscopic residual tumor, microscopic residual tumor, and residual tumor not otherwise specified), indeterminate, or not available/unknown. Margin status was coded as it appeared in the pathology report of the reporting facility.
Multivariable analysis of predictors of PM was performed using a generalized estimating equation model with logit link and exchangeable correlation structure, accounting for patient clustering at facilities and adjusting for the same demographic, clinical, and facility factors as in the univariable analysis. Facility volume was treated as a continuous variable in this analysis. We predicted average marginal effects of facility volume from this model as the probability of PM and plotted this along with true observed PM rates to directly compare unadjusted and adjusted volume-outcome relationships.
To evaluate whether the facility volume effect was influenced by facility type, we computed a multivariable generalized estimating equation model that allowed for an interaction between facility volume and facility type (ie, academic, nonacademic). We then plotted predicted and observed probabilities of PM across facility volume (separately for academic and nonacademic facilities), as previously described.
Finally, we assessed the association of facility volume with the likelihood of receiving adjuvant CRT among patients with PM, using a multivariable generalized estimating equation model. Predicted and observed probabilities of receiving CRT were then plotted across facility volume.
All analysis was performed using Stata, version 14.2 (StataCorp). All tests were 2 sided, and P < .05 was considered statistically significant.
Results
We identified 28 840 patients who met selection criteria. Characteristics of the final cohort are detailed in Table 1. The overall PM rate was 17.6%, and the average age was 62.4 years (range, 40 to ≥90 years). A majority of patients were white (84.6% [n = 24 399]) and had no additional comorbidities (74.0% [n = 21 342]). The most common primary site was the oral cavity (53.7% [n = 15 487]), followed by the oropharynx (26.0% [n = 7498]), larynx (18.8% [n = 5422]), and hypopharynx (1.5% [n = 433]). The average annual facility caseload was 29.0, and the majority of facilities were academic (59.0% [n = 17 016]).
Table 1. Demographic and Clinical Characteristics for Overall Cohort of Patients With HNSCC Treated With Definitive Surgery.
| Characteristic | Study Cohort (n = 28 840) |
|---|---|
| Age, median (IQR), y | 61 (54-70) |
| Sex, No. (%) | |
| Male | 19 727 (68.4) |
| Female | 9113 (31.6) |
| Race/ethnicity, No. (%) | |
| White, non-Hispanic | 24 398 (84.6) |
| Black, non-Hispanic | 2134 (7.4) |
| Hispanic | 1125 (3.9) |
| Asian or Pacific Islander | 721 (2.5) |
| Other or unknown | 490 (1.7) |
| Insurance status, No. (%) | |
| Private | 12 661 (43.9) |
| Medicare or other government insurance | 11 680 (40.5) |
| Medicaid | 2624 (9.1) |
| Unknown or uninsured | 1846 (6.4) |
| Socioeconomic status, No. (%) | |
| Low | 9950 (34.5) |
| Middle | 9373 (32.5) |
| High | 9402 (32.6) |
| Unknown | 115 (0.4) |
| Comorbidity index, No. (%) | |
| 0 | 21 342 (74.0) |
| 1 | 5739 (19.9) |
| ≥2 | 1730 (6.0) |
| Primary site, No. (%) | |
| Oral cavity | 15 487 (53.7) |
| Oropharynx | 7498 (26.0) |
| Larynx | 5422 (18.8) |
| Hypopharynx | 433 (1.5) |
| Tumor grade, No. (%) | |
| 1 | 4932 (17.1) |
| 2 | 14 737 (51.1) |
| 3 | 6604 (22.9) |
| Unknown | 2567 (8.9) |
| Pathologic T category, No. (%) | |
| 1 | 11 507 (39.9) |
| 2 | 7008 (24.3) |
| 3 | 2682 (9.3) |
| 4 | 4701 (16.3) |
| X | 2942 (10.2) |
| Pathologic N category, No. (%) | |
| 0 | 10 469 (36.3) |
| 1 | 3028 (10.5) |
| 2 | 6979 (24.2) |
| 3 | 288 (1.0) |
| X | 8046 (27.9) |
| Clinical TNM stage, No. (%) | |
| I | 9604 (33.3) |
| II | 5220 (18.1) |
| III | 4586 (15.9) |
| IVA | 9200 (31.9) |
| IVB | 260 (0.9) |
| Facility volume by annual cases, median (IQR) | 20.5 (5.4-38.6) |
| Facility type, No. (%) | |
| Academic | 17 016 (59.0) |
| Nonacademic | 11 824 (41.0) |
| Margin status, No. (%) | |
| Negative | 23 764 (82.4) |
| Positive | 5076 (17.6) |
Abbreviations: HNSCC, head and neck squamous cell carcinoma; IQR, interquartile range.
Predictors of PM
Risk ratios (RR) of PM according to patient and facility characteristics are detailed in Table 2. Patients who were younger; male; black; had Medicaid insurance; had primary sites of the oropharynx, hypopharynx, or larynx; and had higher tumor grade or TNM stage were all significantly more likely to have PM. Higher facility volume was associated with lower PM rate (compared with the lowest-volume quartile: RR, 0.63 [95% CI, 0.59-0.66] for the middle 2 quartiles; and RR, 0.41 [95% CI, 0.38-0.44] for the highest-volume quartile). Patients treated at academic facilities had a PM rate of 14%. Among nonacademic facilities, CCPs had a PM rate of 28.2%, CCCPs had 22.8%, and INCPs had 19.3%. Patients treated at academic facilities were significantly less likely to have PM (compared with CCPs with the highest rate of PM: RR, 0.50 [95% CI, 0.45-0.54]).
Table 2. Positive Margin Rate by Demographic and Clinical Characteristics of Patients.
| Positive Margin Rate, No. (%) | Risk Ratio (95% CI) | |
|---|---|---|
| Age, y | ||
| <65 | 5249 (18.2) | 1 [Reference] |
| ≥65 | 4759 (16.5) | 0.91 (0.86-0.96) |
| Sex | ||
| Male | 5508 (19.1) | 1 [Reference] |
| Female | 4066 (14.1) | 0.74 (0.70-0.78) |
| Race/ethnicity | ||
| White | 5047 (17.5) | 1 [Reference] |
| Black | 5595 (19.4) | 1.11 (1.01-1.21) |
| Hispanic | 4960 (17.2) | 0.98 (0.86-1.11) |
| Asian or Pacific Islander | 4038 (14.0) | 0.80 (0.66-0.96) |
| Other or unknown | 4730 (16.4) | 0.93 (0.76-1.15) |
| Insurance status | ||
| Private | 5105 (17.7) | 1 [Reference] |
| Medicare or other government insurance | 4932 (17.1) | 0.97 (0.92-1.02) |
| Medicaid | 5537 (19.2) | 1.09 (1.00-1.19) |
| Unknown or uninsured | 4932 (17.1) | 0.97 (0.87-1.08) |
| Socioeconomic status | ||
| Low | 5047 (17.5) | 1 [Reference] |
| Middle | 5220 (18.1) | 1.04 (0.98-1.10) |
| High | 4932 (17.1) | 0.98 (0.92-1.04) |
| Unknown | 4441 (15.4) | 0.88 (0.58-1.34) |
| Comorbidity index | ||
| 0 | 5018 (17.4) | 1 [Reference] |
| 1 | 5162 (17.9) | 1.03 (0.97-1.10) |
| ≥2 | 5249 (18.2) | 1.04 (0.94-1.16) |
| Primary site | ||
| Oral cavity | 3115 (10.8) | 1 [Reference] |
| Oropharynx | 8508 (29.5) | 2.74 (2.58-2.90) |
| Hypopharynx | 7787 (27.0) | 2.51 (2.13-2.94) |
| Larynx | 5681 (19.7) | 1.83 (1.70-1.96) |
| Tumor grade | ||
| 1 | 2682 (9.3) | 1 [Reference] |
| 2 | 4960 (17.2) | 1.84 (1.68-2.03) |
| 3 | 7181 (24.9) | 2.68 (2.43-2.95) |
| Unknown | 4816 (16.7) | 1.79 (1.58-2.03) |
| Pathologic T category | ||
| 1 | 3201 (11.1) | 1 [Reference] |
| 2 | 5480 (19.0) | 1.71 (1.59-1.83) |
| 3 | 5797 (20.1) | 1.81 (1.65-1.98) |
| 4 | 5681 (19.7) | 1.77 (1.64-1.92) |
| X | 9690 (33.6) | 3.03 (2.82-3.25) |
| Pathologic N category | ||
| 0 | 2596 (9.0) | 1 [Reference] |
| 1 | 4960 (17.2) | 1.92 (1.74-2.11) |
| 2 | 6201 (21.5) | 2.39 (2.22-2.58) |
| 3 | 8018 (27.8) | 3.09 (2.54-3.76) |
| X | 7239 (25.1) | 2.79 (2.60-3.00) |
| Clinical TNM stage | ||
| I | 2653 (9.2) | 1 [Reference] |
| II | 4816 (16.7) | 1.81 (1.66-1.98) |
| III | 6345 (22.0) | 2.39 (2.20-2.60) |
| IVA | 6922 (24.0) | 2.61 (2.43-2.81) |
| IVB | 10 296 (35.7) | 3.88 (3.25-4.62) |
| Facility volume | ||
| Low (<25th percentile) | 7585 (26.3) | 1 [Reference] |
| Medium (25th-75th percentile) | 4759 (16.5) | 0.63 (0.59-0.66) |
| High (>75th percentile) | 3115 (10.8) | 0.41 (0.38-0.44) |
| Facility type | ||
| Nonacademic | ||
| CCP | 8133 (28.2) | 1 [Reference] |
| CCCP | 6576 (22.8) | 0.81 (0.74-0.89) |
| INCP | 5566 (19.3) | 0.68 (0.61-0.76) |
| Academic | 4038 (14.0) | 0.50 (0.45-0.54) |
| Facility location | ||
| New England | 6489 (22.5) | 1 [Reference] |
| Middle Atlantic | 4124 (14.3) | 0.63 (0.56-0.72) |
| South Atlantic | 5451 (18.9) | 0.84 (0.75-0.94) |
| East North Central | 4845 (16.8) | 0.75 (0.67-0.84) |
| East South Central | 4816 (16.7) | 0.74 (0.65-0.85) |
| West North Central | 4701 (16.3) | 0.72 (0.63-0.82) |
| West South Central | 4845 (16.8) | 0.75 (0.65-0.86) |
| Mountain | 5797 (20.1) | 0.89 (0.77-1.03) |
| Pacific | 5739 (19.9) | 0.89 (0.78-1.00) |
Abbreviations: CCP, community cancer program; CCCP, comprehensive community cancer program; INCP, integrated network cancer program.
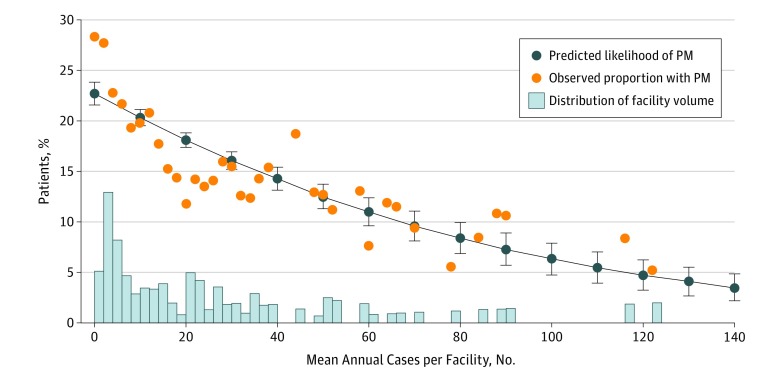
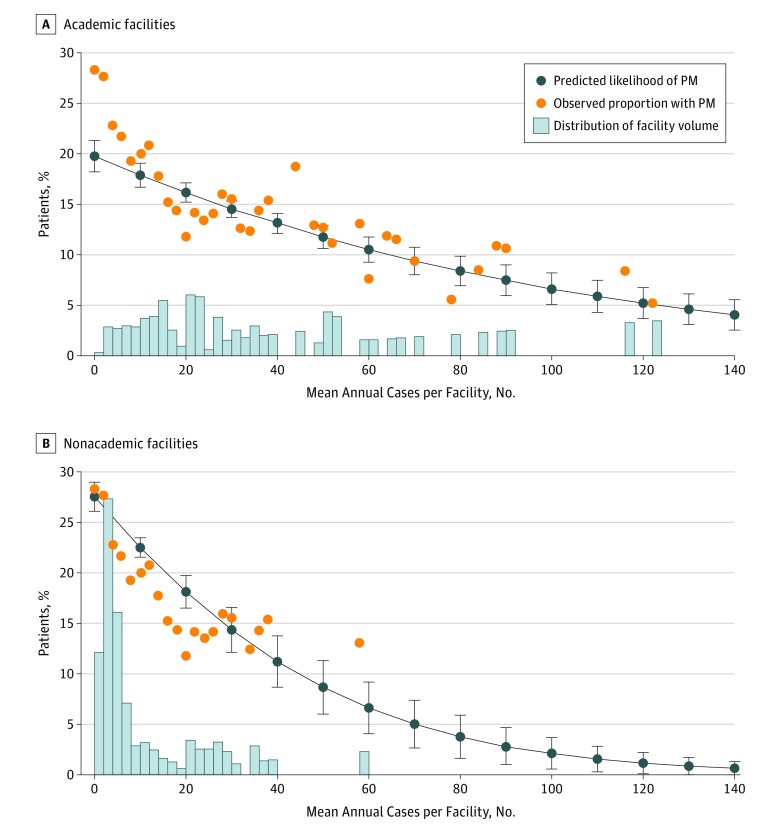
In multivariable analysis, those treated at higher-volume facilities remained significantly less likely to have PM (Figure 1) (adjusted odds ratio [aOR], 0.85 per 10-case volume increase [95% CI, 0.83-0.88]). Importantly, the predicted likelihood of PM decreased with increasing facility volume and closely matched the observed likelihood of PM. The predicted and observed likelihoods were approximately 20% for patients at facilities with 10 annual cases and as low as 10% at facilities with 70 annual cases. The trend of decreasing PM rate with increasing facility volume was observed in both academic (Figure 2A) (aOR, 0.88 per 10-case volume increase [95% CI, 0.85-0.91]) and nonacademic (Figure 2B) (aOR, 0.73 per 10-case volume increase [95% CI, 0.68-0.80]) facilities, despite substantial differences in the distributions of volume between these types of facilities. There was no association between facility volume and PM among the different types of nonacademic facilities (compared with CCPs: aOR, 0.98 per 10-case volume increase [95% CI, 0.84-1.14] for CCCPs; and aOR, 1.24 [95% CI, 0.99-1.55] for INCPs).
Figure 1. Treatment at Higher-Volume Facilities.
Facility head and neck cancer resection volume and likelihood of positive margin (PM) predicted from a multivariable generalized estimating equation model (dark blue line with markers). Results overlaid on observed proportion of patients with PM according to facility volume (orange circles) and histogram showing distribution of facility volume by across the cohort (light blue bars).
Figure 2. Trend in Decreasing Positive Margin Rate and Increasing Facility Volume.
Facility head and neck cancer resection volume and likelihood of positive margin (PM) stratified by facility type and predicted from a multivariable generalized estimating equation model (dark blue lines with markers). Results overlaid on observed proportion of patients with PM according to facility volume (orange circles) and histogram showing distribution of facility volume across the cohort (blue bars).
Receipt of Adjuvant Chemoradiation Therapy for PM
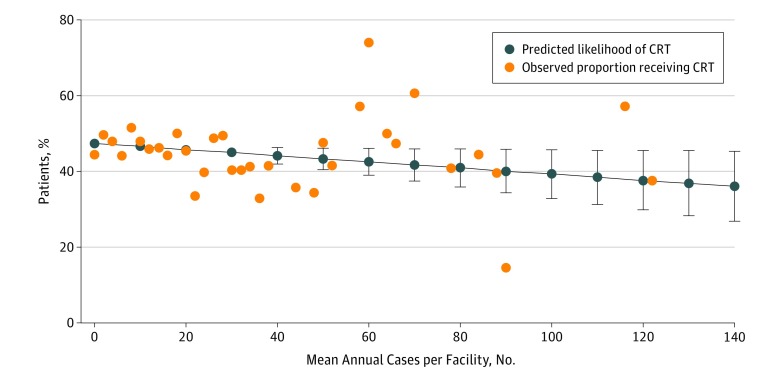
In contrast, we observed no association between facility volume and patient likelihood of receiving adjuvant CRT in the setting of PM (Figure 3) (aOR, 0.96 per 10-case volume increase [95% CI, 0.91-1.00]). This was true among both the observed data and the probabilities predicted by the multivariable model. Notably, the predicted likelihood of receiving CRT in the setting of PM was less than 50% regardless of facility volume.
Figure 3. Facility Volume and Patient Likelihood of Receiving Adjuvant Chemoradiation Therapy.
Facility head and neck cancer resection volume and likelihood of receiving adjuvant chemoradiation therapy (CRT) among patients with positive surgical margins. The results are the predicted likelihood from a multivariable generalized estimating equation model (dark blue lines with markers) and overlaid on observed proportion of patients receiving CRT according to facility volume (orange circles).
Discussion
We found that surgical treatment for HNSCC at a low-volume facility was associated with an increased likelihood of PM. This association persisted whether surgery was performed at an academic or nonacademic facility, despite overall volume differences between these 2 types of facilities. We found no association between facility volume and the use of adjuvant CRT in the setting of PM.
The present study focused on the influence of volume on the surgical outcome of PM, a clear measure of surgical success and patient prognosis. The findings of this study contribute to the growing body of literature examining the association between surgical volume and outcomes in head and neck oncology.17 In studies limited to single head and neck subsites, high-volume hospitals and surgeons have been found to be associated with improved survival, shorter hospitalization stays, lower cost of care, and lower complication rates.8,18,19,20,21 It has been suggested that high-volume facilities and surgeons benefit from the coordination of a large, experienced team of specialists who are properly equipped and trained to care for patients with complex head and neck cancer.8
Surgical Volume and PM
Among the high-risk features in HNSCC, PM is the only one in which the surgeon has some control, independent of facility factors. Previously published studies examining the relationship between volume and PM have only included oral cavity cancers.12,13,22 A recent study by Ellis et al12 on oral cavity cancer found that higher-volume surgeons delivered larger surgical margins than lower-volume surgeons and that surgeon volume had the single greatest influence on surgical margin dimensions. This has important clinical implications because, according to NCCN guidelines, surgery is the recommended primary treatment modality for oral cavity cancer.1 In contrast to that study, which included only 250 patients with oral cancer from a single institution, the present study included nearly 30 000 patients with HNSCC from a national database. An older oral cancer study found a close association between a close margin or PM and histological indicators of aggressive disease, although not with the treating surgeon.22 However, that study only included 2 surgeons and did not define their caseloads. The authors argued that a close margin or PM should be considered “a product of aggressive tumor behavior in addition to, or even rather than, inadequate surgical resection.”22(p34) However, aggressive tumor biology should not be a reason to forgo a more radical surgery or deem a patient unresectable, especially with the reconstructive options currently available to help reduce the cosmetic and functional morbidity of such resections.
A similar NCDB analysis of PM in localized stage oral cancers (stage I/II) was performed by Luryi et al.13 The authors found that treatment at low-volume facilities and at nonacademic facilities was associated with an independent increase in the risk of PM. Treatment at facilities reporting fewer than 20 cases per year was associated with a 30% increase in incidence of PM compared with those reporting more than 20 cases per year. Similarly, treatment at nonacademic facilities was associated with a 23% increase in PM compared with academic facilities. However, that study was limited to only localized stage oral cavity cancer from an older data set. The present study adds to the current literature by using a large, contemporary data set and including all TNM stages and head and neck subsites.
Positive Margin and Guidelines Adherence
The present study found that facility volume was not associated with the use of adjuvant CRT in the setting of PM. We demonstrated that the overall likelihood of receiving CRT was approximately 50%. The use of adjuvant CRT in the setting of PM following surgery for HNSCC is a category 1 recommendation according to NCCN guidelines (except for oropharynx primary cancer, which is a category 2a recommendation).1 Moreover, a recent analysis of the NCDB by our group confirmed a significant survival benefit with the use of adjuvant CRT in patients who have PM after surgery.23 Our CRT analysis is exploratory and did not evaluate its administration by site or stage, but our findings highlight concerns over guideline adherence in the management of head and neck cancer and requires further investigation.
Lewis et al24 found that prereferral care among patients referred to the University of Texas MD Anderson Cancer Center, a high-volume, tertiary care center, was noncompliant with NCCN guidelines in 43% of patients. Remarkably, 10.9% received inadequate adjuvant therapy. In contrast, Hessel et al25 found an overall very high rate of guideline adherence at MD Anderson Cancer Center, with 98% of patients appropriately referred to the Radiation Oncology division when high-risk features on pathology were identified. In their population-based analysis, Eskander et al26 found that higher hospital and surgeon volumes for head and neck cancer were associated with higher rates of adherence to guideline-recommended processes of care. Such findings underscore the potential value of highly specialized referral centers with high caseloads for the proper management of HNSCC.
Other Factors Associated With PM
The availability of frozen sections has been evaluated for its potential influence on surgical margins. Frozen sections provide preliminary margin status intraoperatively with the goal of achieving definitive clear margins. Although frozen sections are highly accurate in the evaluation of margins for HNSCC,27 the selection of representative margins for intraoperative analysis—whether from the tumor bed or main specimen—and its effect on recurrence and survival is still an area of contention.28,29 It also adds to the operative time and incurs extra service costs. The availability of frozen sections during the surgical resection of HNSCC could theoretically lower the rate of PM, but there is conflicting evidence in the literature that neither supports nor refutes this relationship.12
The availability of a reconstructive surgeon, with the ability to perform microvascular free flap reconstruction, is another potential factor that can influence margin status. It can be hypothesized that the availability of microvascular free tissue transfer allows for a more aggressive tumor resection to achieve clear margins, despite the potential creation of extensive functional and cosmetic defects. In 2 small retrospective studies on surgically resectable oral cavity squamous cell carcinoma,12,30 no association was found between the presence of PM and reconstruction with free tissue transfer, as opposed to a locoregional flap. A large retrospective study on only T3 and T4 category oral cancers,31 however, showed a significant decrease in the rate of PM (from 18% to 7%) after the introduction of routine free flap reconstruction. It may be that the larger ablative surgeries required for locally advanced HNSCC are more likely to be limited in scope if free flap reconstruction is not available. Such may be the scenario at lower-volume hospitals where the low caseload does not attract or warrant the hiring of reconstructive surgeons, or justify supporting patients’ complex postoperative care needs.
Limitations
The availability of intraoperative margin analysis and a reconstructive surgeon are possible confounding factors that are not included in our analysis, because these datapoints are not available in the NCDB. There are several other important limitations to our work. As a retrospective observational study, it is difficult to draw any causal conclusions about observed associations. The NCDB is also a facility-based data set that only captures cancers diagnosed at CoC-accredited facilities, which may not reflect trends in oncologic outcomes for non–CoC-accredited facilities. Importantly, there is no universally accepted definition of what constitutes a negative, close, or positive margin, which makes it challenging to compare surgical outcomes among different facilities.6 This ambiguity can lead to database coding errors and can bias the choice of adjuvant therapy at individual treating centers. The margin status reported in the NCDB is taken from the pathology report from the reporting facility. It is not necessarily reflective of the margin status as determined by the surgeon or from a multidisciplinary tumor board discussion, where decisions regarding adjuvant therapy are often made. Finally, we are unable to evaluate the relationship between PM and surgeon volume, treated separately from hospital volume, as this information is not available in the NCDB.
Conclusions
These findings suggest that high-volume facilities have lower rates of PM in the surgical treatment of HNSCC in both the academic and nonacademic settings. Despite this discrepancy between high- and low-volume facilities, there is no corresponding difference in the use of guideline-recommended adjuvant CRT. This study underscores the benefits of high-volume HNSCC centers on surgical outcomes and should be a consideration when deciding where to refer, or receive, cancer care. Positive margin rates and facility volume for head and neck oncologic surgeries may be considered a benchmark for quality of care.
References
- 1.Pfister DG, Spencer S, Brizel DM, et al. ; National Comprehensive Cancer Network . Head and neck cancers, version 2.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12(10):1454-1487. doi: 10.6004/jnccn.2014.0142 [DOI] [PubMed] [Google Scholar]
- 2.Rich JT, Milov S, Lewis JS Jr, Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) +/− adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119(9):1709-1719. doi: 10.1002/lary.20552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haque R, Contreras R, McNicoll MP, Eckberg EC, Petitti DB. Surgical margins and survival after head and neck cancer surgery. BMC Ear Nose Throat Disord. 2006;6:2. doi: 10.1186/1472-6815-6-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eldeeb H, Macmillan C, Elwell C, Hammod A. The effect of the surgical margins on the outcome of patients with head and neck squamous cell carcinoma: single institution experience. Cancer Biol Med. 2012;9(1):29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smits RWH, Koljenović S, Hardillo JA, et al. . Resection margins in oral cancer surgery: room for improvement. Head Neck. 2016;38(suppl 1):E2197-E2203. doi: 10.1002/hed.24075 [DOI] [PubMed] [Google Scholar]
- 6.Amit M, Yen TC, Liao CT, et al. ; International Consortium for Outcome Research in Head and Neck Cancer . Improvement in survival of patients with oral cavity squamous cell carcinoma: an international collaborative study. Cancer. 2013;119(24):4242-4248. doi: 10.1002/cncr.28357 [DOI] [PubMed] [Google Scholar]
- 7.Zhang SY, Lu ZM, Luo XN, et al. . Retrospective analysis of prognostic factors in 205 patients with laryngeal squamous cell carcinoma who underwent surgical treatment. PLoS One. 2013;8(4):e60157. doi: 10.1371/journal.pone.0060157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CC, Ho HC, Chou P. Multivariate analyses to assess the effect of surgeon volume on survival rate in oral cancer: a nationwide population-based study in Taiwan. Oral Oncol. 2010;46(4):271-275. doi: 10.1016/j.oraloncology.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 9.Eskander A, Irish J, Groome PA, et al. . Volume-outcome relationships for head and neck cancer surgery in a universal health care system. Laryngoscope. 2014;124(9):2081-2088. doi: 10.1002/lary.24704 [DOI] [PubMed] [Google Scholar]
- 10.Lassig AAD, Joseph AM, Lindgren BR, et al. . The effect of treating institution on outcomes in head and neck cancer. Otolaryngol Head Neck Surg. 2012;147(6):1083-1092. doi: 10.1177/0194599812457324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George JR, Yom SS, Wang SJ. Improved outcomes in adjuvant radiotherapy for oral cavity carcinoma at an academic center: a matched-pair analysis. Laryngoscope. 2014;124(7):1603-1608. doi: 10.1002/lary.24552 [DOI] [PubMed] [Google Scholar]
- 12.Ellis OG, David MC, Park DJ, Batstone MD. High-volume surgeons deliver larger surgical margins in oral cavity cancer. J Oral Maxillofac Surg. 2016;74(7):1466-1472. doi: 10.1016/j.joms.2016.01.026 [DOI] [PubMed] [Google Scholar]
- 13.Luryi AL, Chen MM, Mehra S, Roman SA, Sosa JA, Judson BL. Positive surgical margins in early stage oral cavity cancer: an analysis of 20,602 cases. Otolaryngol Head Neck Surg. 2014;151(6):984-990. doi: 10.1177/0194599814551718 [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683-690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer Publishing; 2011. [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 17.Eskander A, Merdad M, Irish JC, et al. . Volume-outcome associations in head and neck cancer treatment: a systematic review and meta-analysis. Head Neck. 2014;36(12):1820-1834. doi: 10.1002/hed.23498 [DOI] [PubMed] [Google Scholar]
- 18.Gourin CG, Frick KD. National trends in laryngeal cancer surgery and the effect of surgeon and hospital volume on short-term outcomes and cost of care. Laryngoscope. 2012;122(1):88-94. doi: 10.1002/lary.22409 [DOI] [PubMed] [Google Scholar]
- 19.Gourin CG, Frick KD. National trends in oropharyngeal cancer surgery and the effect of surgeon and hospital volume on short-term outcomes and cost of care. Laryngoscope. 2012;122(3):543-551. doi: 10.1002/lary.22447 [DOI] [PubMed] [Google Scholar]
- 20.Jalisi S, Bearelly S, Abdillahi A, Truong MT. Outcomes in head and neck oncologic surgery at academic medical centers in the United States. Laryngoscope. 2013;123(3):689-698. doi: 10.1002/lary.23835 [DOI] [PubMed] [Google Scholar]
- 21.Lee CC, Ho HC, Jack LC, et al. . Association between surgeon volume and hospitalisation costs for patients with oral cancer: a nationwide population base study in Taiwan. Clin Otolaryngol. 2010;35(1):46-52. doi: 10.1111/j.1749-4486.2009.02071.x [DOI] [PubMed] [Google Scholar]
- 22.Sutton DN, Brown JS, Rogers SN, Vaughan ED, Woolgar JA. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2003;32(1):30-34. doi: 10.1054/ijom.2002.0313 [DOI] [PubMed] [Google Scholar]
- 23.Ajmani GS, Nocon CC, Wang CH, Bhayani MK. Assessment of adjuvant therapy in resected head and neck cancer with high-risk features. Oral Oncol. 2017;74:15-20. doi: 10.1016/j.oraloncology.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 24.Lewis CM, Hessel AC, Roberts DB, et al. . Prereferral head and neck cancer treatment: compliance with national comprehensive cancer network treatment guidelines. Arch Otolaryngol Head Neck Surg. 2010;136(12):1205-1211. doi: 10.1001/archoto.2010.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hessel AC, Moreno MA, Hanna EY, et al. . Compliance with quality assurance measures in patients treated for early oral tongue cancer. Cancer. 2010;116(14):3408-3416. doi: 10.1002/cncr.25031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eskander A, Monteiro E, Irish J, et al. . Adherence to guideline-recommended process measures for squamous cell carcinoma of the head and neck in Ontario: impact of surgeon and hospital volume. Head Neck. 2016;38(suppl 1):E1987-E1992. doi: 10.1002/hed.24364 [DOI] [PubMed] [Google Scholar]
- 27.Layfield EM, Schmidt RL, Esebua M, Layfield LJ. Frozen section evaluation of margin status in primary squamous cell carcinomas of the head and neck: a correlation study of frozen section and final diagnoses. Head Neck Pathol. 2017;12(2):175-180. doi: 10.1007/s12105-017-0846-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maxwell JH, Thompson LD, Brandwein-Gensler MS, et al. . Early oral tongue squamous cell carcinoma: sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1104-1110. doi: 10.1001/jamaoto.2015.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchakjian MR, Tasche KK, Robinson RA, Pagedar NA, Sperry SM. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016;142(12):1191-1198. doi: 10.1001/jamaoto.2016.2329 [DOI] [PubMed] [Google Scholar]
- 30.de Vicente JC, Rodríguez-Santamarta T, Rosado P, Peña I, de Villalaín L. Survival after free flap reconstruction in patients with advanced oral squamous cell carcinoma. J Oral Maxillofac Surg. 2012;70(2):453-459. doi: 10.1016/j.joms.2011.02.020 [DOI] [PubMed] [Google Scholar]
- 31.Hanasono MM, Friel MT, Klem C, et al. . Impact of reconstructive microsurgery in patients with advanced oral cavity cancers. Head Neck. 2009;31(10):1289-1296. doi: 10.1002/hed.21100 [DOI] [PubMed] [Google Scholar]