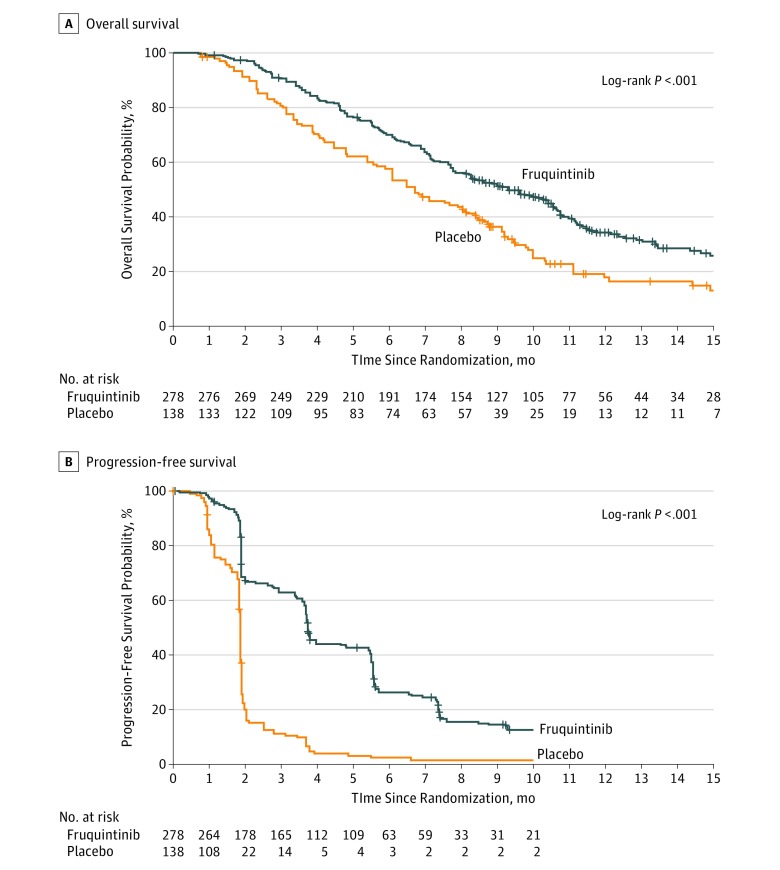
Figure 2. Kaplan-Meier Estimates for Overall Survival and Progression-Free Survival in Patients With Metastatic Colorectal Cancer Receiving Fruquintinib vs Placebo (Intent-to-Treat Population).
All eligible patients had Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (0 indicates fully active, able to carry on all predisease activities without restriction; 1 indicates restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature [eg, light housework, office work]). The median follow-up time was 13.3 months (95% CI, 12.1-14.7) for the fruquintinib group and 13.2 months (95% CI, 10.6-19.6) for the placebo group. Tick marks on the curves denote the last known follow-up time for patients with no death date reported.
A, The hazard ratio (HR) for death and corresponding 95% CI for overall population were estimated from a stratified Cox proportional hazards model. Stratified factors included use of vascular endothelial growth factor (VEGF) inhibitor (yes vs no) and K-ras gene state (wild type vs mutated). At the planned cutoff date (January 17, 2017), after 297 deaths, the median overall survival was 9.3 (95% CI, 8.2-10.5) months in the fruquintinib group and 6.6 (95% CI, 5.9-8.1) months in the placebo group (HR for death, 0.65 [95% CI, 0.51-0.83]; log-rank P < .001).
B, The HR for progression-free survival and corresponding 95% CI were estimated from stratified Cox proportional hazards model with treatment as the only covariate. Stratified factors included use of VEGF inhibitors and K-ras gene status. Median progression-free survival was also significantly increased with fruquintinib (3.7 months [95% CI, 3.7-4.6] vs 1.8 [95% CI, 1.8-1.8] months with placebo; HR for progression or death, 0.26 [95% CI, 0.21-0.34]; P < .001).