Key Points
Question
Does drinking more water protect against declining kidney function in patients with chronic kidney disease?
Findings
In this randomized clinical trial that included 631 adult patients with chronic kidney disease, the 1-year decline in estimated glomerular filtration rate did not significantly differ between patients who were coached to drink more water compared with patients who were coached to maintain their usual intake (−2.2 vs −1.9 mL/min per 1.73 m2).
Meaning
Coaching to increase water intake did not significantly slow the decline in kidney function in patients with chronic kidney disease at 1-year follow-up.
Abstract
Importance
In observational studies, increased water intake is associated with better kidney function.
Objective
To determine the effect of coaching to increase water intake on kidney function in adults with chronic kidney disease.
Design, Setting, and Participants
The CKD WIT (Chronic Kidney Disease Water Intake Trial) randomized clinical trial was conducted in 9 centers in Ontario, Canada, from 2013 until 2017 (last day of follow-up, May 25, 2017). Patients had stage 3 chronic kidney disease (estimated glomerular filtration rate [eGFR] 30-60 mL/min/1.73 m2 and microalbuminuria or macroalbuminuria) and a 24-hour urine volume of less than 3.0 L.
Interventions
Patients in the hydration group (n = 316) were coached to drink more water, and those in the control group (n = 315) were coached to maintain usual intake.
Main Outcomes and Measures
The primary outcome was change in kidney function (eGFR from baseline to 12 months). Secondary outcomes included 1-year change in plasma copeptin concentration, creatinine clearance, 24-hour urine albumin, and patient-reported overall quality of health (0 [worst possible] to 10 [best possible]).
Results
Of 631 randomized patients (mean age, 65.0 years; men, 63.4%; mean eGFR, 43 mL/min/1.73 m2; median urine albumin, 123 mg/d), 12 died (hydration group [n = 5]; control group [n = 7]). Among 590 survivors with 1-year follow-up measurements (95% of 619), the mean change in 24-hour urine volume was 0.6 L per day higher in the hydration group (95% CI, 0.5 to 0.7; P < .001). The mean change in eGFR was −2.2 mL/min/1.73 m2 in the hydration group and −1.9 mL/min/1.73 m2 in the control group (adjusted between-group difference, −0.3 mL/min/1.73 m2 [95% CI, −1.8 to 1.2; P = .74]). The mean between-group differences (hydration vs control) in secondary outcomes were as follows: plasma copeptin, −2.2 pmol/L (95% CI, −3.9 to −0.5; P = .01); creatinine clearance, 3.6 mL/min/1.73 m2 (95% CI, 0.8 to 6.4; P = .01); urine albumin, 7 mg per day (95% CI, −4 to 51; P = .11); and quality of health, 0.2 points (95% CI, −0.3 to 0.3; P = .22).
Conclusions and Relevance
Among adults with chronic kidney disease, coaching to increase water intake compared with coaching to maintain the same water intake did not significantly slow the decline in kidney function after 1 year. However, the study may have been underpowered to detect a clinically important difference.
Trial Registration
clinicaltrials.gov Identifier: NCT01766687.
This randomized clinical trial compares the effects of coaching patients with chronic kidney disease to increase vs maintain or reduce their free water intake on change in their estimated glomerular filitration rate at 12 months.
Introduction
Despite widespread interest, little scientific data exist on the optimal amount of water to drink. The popular advice to “drink at least 8 glasses of water a day” originated not from primary research, but from the US Food and Nutrition Board in 1945, which recommended a daily water intake of 2.5 L per day and stated that the majority of this intake could come from food sources.1,2 While many claims about the benefits of increased water intake remain untested,2,3,4 a growing body of evidence suggests that drinking more water may benefit the kidneys.5,6 In animals that undergo a near complete bilateral nephrectomy, drinking more water suppresses the plasma concentration of vasopressin and improves creatinine clearance,7,8 and in studies of humans, drinking more water is associated with better kidney function and a reduced risk of kidney stones.9,10,11,12,13 Whether increased water intake can benefit patients with chronic kidney disease remains unknown.
In a 6-week pilot randomized clinical trial of 29 patients with stage 3 chronic kidney disease, coaching patients to drink at least 1 additional liter of water per day (in addition to usual fluid intake) resulted in reduced vasopressin secretion (as assessed by the plasma concentration of copeptin, a glycosylated peptide that is co-released with vasopressin from the hypothalamus) compared with patients in the control group who were coached to maintain usual fluid intake.14,15 Guided by this pilot study, the present 9-center Chronic Kidney Disease Water Intake Trial (CKD WIT) was conducted to determine if coaching patients to drink more water slowed their decline in kidney function over 1 year compared with those in the control group who were coached to maintain usual fluid intake.
Methods
Study Design and Oversight
A parallel-group randomized clinical trial was conducted from April 2013 to June 2017. Ethical approval to conduct this trial was obtained from Western University’s health sciences research ethics board. All patients provided written informed consent. The original protocol (Supplement 1) and the statistical analysis plan (Supplement 2) for this trial are available online, the updated protocol was published previously,16 and changes to the original protocol are summarized in eTable 1 in Supplement 3. Prespecified and post hoc outcomes are summarized in eTable 2 in Supplement 3 along with the date that each outcome was added. Study reporting follows recommended guidelines for randomized trials of nonpharmacologic treatments.17
Trial conduct and safety were monitored by an independent data and safety monitoring board, which received a descriptive summary of trial data and adverse events at intervals of 3 to 9 months, with no planned interim statistical analysis of the primary or secondary outcomes. The data and safety monitoring board requested and received access to data on estimated glomerular filtration rate (eGFR) and group assignment but did not discuss these data with the trial investigators.
Patients, Setting, and Location
Adult patients with stage 3 chronic kidney disease were recruited from 9 Canadian chronic kidney disease clinics across southwestern Ontario: London (3 centers), Oakville (2 centers), Windsor (2 centers), Guelph (1 center), and Hamilton (1 center). Potentially eligible patients were invited by their nephrologist to speak with a research assistant who explained the study, confirmed eligibility (eTable 3 in Supplement 3), and obtained written informed consent. Inclusion criteria were stage 3 chronic kidney disease (eGFR 30 to 60 mL/min per 1.73 m2) and an albumin-to-creatinine ratio of greater than 25 mg/g (2.8 mg/mmol) for women or greater than 18 mg/g (2.0 mg/mmol) for men (from a random spot urine sample) or trace protein or greater (from a urine dipstick).18 Exclusion criteria included self-reported fluid intake of 10 cups per day or more, a 24-hour urine volume of 3 L or more, a history of kidney stones in the past 5 years, currently taking lithium or a diuretic above a certain dose (eTable 3 in Supplement 3), or a prescribed fluid restriction of less than 1.5 L per day.
After providing written informed consent, patients provided a prerandomization 24-hour urine sample to confirm their urine volume was less than 3 L per day (which was an eligibility requirement for randomization).
Randomized Allocation
Once study eligibility was confirmed, a research assistant contacted each patient by telephone to complete the randomization, and concealed randomized allocation was implemented by computer-generated randomization while the patient was on the phone. The randomized sequence was created by an independent statistician using SAS statistical software version 9.3 and was stratified by center and sex with a 1:1 allocation using random permuted block sizes of 4 and 6.
After randomization, research staff and patients were made aware of group assignment; however, the primary outcome was an objective measure, outcome assessors (technicians performing the laboratory measurements for the primary and secondary outcomes) were unaware of the random allocation, and the trial statistician was blinded to patient allocation for the primary analysis.
Intervention Group
Patients in the hydration group were coached to increase their water intake by 1.0 to 1.5 L per day (depending on sex and weight), over and above usual consumed beverages, for the duration of the 1-year trial (eTable 4 in Supplement 3). The target water intake was informed by prior literature,9,19,20,21 patient safety, and what proved feasible in the pilot trial.14,15 A gradual increase in water intake over 2 weeks was advised. During week 1, patients were instructed to drink an additional cup of water at breakfast, lunch, and dinner and to increase to the full amount in week 2. Patients in the hydration group also received reusable drinking containers and were mailed 20 vouchers per month, each redeemable for 1.5 L of bottled water.
Control Group
Patients in the control group were coached to continue with their usual fluid intake or to decrease intake by 0.25 to 0.5 L per day (1 to 2 cups/d) if their prerandomization 24-hour urine volume was greater than 1.5 L per day and 24-hour urine osmolality was less than 500 mOsm/kg.14
Intervention Adherence and Monitoring
To encourage adherence to the assigned intervention and to minimize cross-group contamination, patients in the hydration and control groups were individually coached over the phone at monthly intervals for 12 months after randomization using a standardized survey with reference to quantity of fluid ingested relative to the target intake. Coaching also included a discussion of urine color charts (showing the spectrum of diluted to concentrated urine). Intervention adherence was assessed by 24-hour urine volume (collected at 6 and 12 months after randomization and measured by a local laboratory). Adherence was also assessed by self-reported fluid intake (measured at 3, 6, and 9 months after randomization).
Data Collection and Measurement
All patients, irrespective of their randomized group assignment, had the same data collection and measurement schedule (eTable 5 in Supplement 3); more information is provided in Supplement 1 and in the published protocol.16 Serum creatinine was measured using the isotope dilution mass spectroscopy–traceable enzymatic method, and the eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.22 Plasma copeptin concentration was measured from 150-μL blood samples at enrollment (prerandomization), at the patients’ next kidney care clinic visit (usually 6 to 7 months after enrollment), and at 12 months after randomization; these samples were stored at −80°C and analyzed in batches using the automated immunofluorescent Copeptin proAVP assay on the KRYPTOR compact PLUS (BRAHMS GmbH).23 Albuminuria was measured from a 24-hour urine sample using a standard collection jug within 2 weeks of enrollment (prerandomization) and again at 6 and 12 months after randomization. Patient-reported quality of health was measured on a 10-point Likert scale (0 [worst possible] to 10 [best possible]; item 22 from the RAND Kidney Disease Quality of Life Short Form).24,25
Outcomes
The prespecified primary outcome was 1-year change in eGFR from enrollment (prerandomization) to 12 months after randomization.26,27 Prespecified secondary outcomes were 1-year change in plasma copeptin concentration,28,29 creatinine clearance, 24-hour urine albumin,30 and patient-reported overall quality of health (Supplement 1 and the published protocol16).24,25
Other exploratory outcomes (some prespecified and some post hoc; eTable 2 in Supplement 3) were 1-year change in 24-hour urine osmolality, creatinine, urea, sodium, and potassium; serum osmolality, creatinine, and urea; blood pressure, body weight, waist circumference, and body mass index (calculated as weight in kilograms divided by height in meters squared); and the 6-month change in dietary intake of protein and sodium.
Sample Size
In a previous longitudinal study of healthy adults free of chronic kidney disease, those with urine volumes of 3 L per day or more had a significantly slower eGFR decline than those with smaller urine volumes (difference, 0.6 mL/min per 1.73 m2 per year; P = .01).9 Given that in previous studies of patients with chronic kidney disease the mean decline in eGFR ranged from 1.5 to 3.5 mL/min per 1.73 m2 (SD range, 3-5) per year,31,32,33 a minimal clinically important difference of 1 mL/min per 1.73 m2 was prespecified (a small to moderate effect size) and the target enrollment was 700 patients (350 per group; assuming a standard deviation of <5; 2-sided α = .05; power, 80%).
After 3.1 years of recruitment (1.3 years longer than originally planned), 631 patients were randomized. Enrollment was stopped at this time in order to stay within the study’s allocated budget (the trial took longer to conduct and cost was more than originally estimated due to a higher than expected screen failure rate and the need to expand into 7 additional centers [eTable 1 in Supplement 3]). The investigators made this decision in consultation with the data and safety monitoring board to ensure that patients’ contribution to this trial would not be compromised by stopping the trial just short of the enrollment target. One year after the last patient was randomized, 12-month eGFR values for 590 patients (295 per group) were available, which enabled detection of a between-group difference of at least 2 mL/min per 1.73 m2 in the mean change in eGFR over 1 year (based on an observed standard deviation of 9 [2-sided α = .05; power, 80%]). No interim analyses of primary or secondary outcomes were performed.
Analysis
Continuous variables are summarized as mean (SD) or as median (interquartile range [IQR]) as appropriate. No statistical tests were used to compare baseline characteristics.34 For the analysis of the primary outcome, the between-group difference in eGFR change (hydration minus control) was estimated using linear regression with adjustment for the following prespecified covariates measured at baseline: age (in years), sex, obesity (body mass index ≥30), current smoker (yes/no), presence of diabetes, and 24-hour urine albumin (mg/d; log-transformed); and use of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, diuretic, β-blocker, calcium channel blocker, or statin.35 Missing baseline data occurred for less than 0.2% of categorical covariates (if missing, the condition was considered absent) and was 6% for 24-hour urine albumin (imputed using fully conditionally specified models36 [eAppendix 1 in Supplement 3]). Patients who died within 1 year of follow-up were excluded from the primary and secondary outcome analyses (12 of 631 patients [1.9%]). Less than 5% of survivors were missing a 12-month eGFR value (±4 months); in the primary analysis only, missing eGFR data were imputed using fully conditionally specified models36 (eAppendix 1 in Supplement 3).
Several supplementary analyses of eGFR change were conducted using nonimputed data: (1) eGFR measured with cystatin C; (2) a longitudinal analysis of change in eGFR over time; (3) a mixed-effects analysis of eGFR change with a random intercept to account for center; (4) the annual percentage change ([final eGFR−baseline eGFR]÷baseline eGFR); and (5) the proportion of patients with a 1-year eGFR decline of at least 20%.37 To examine the effect of intervention adherence, a prespecified per-protocol analysis was conducted that excluded the following: (1) patients in the hydration group who did not maintain a 24-hour urine volume of at least 0.5 L per day above their baseline value at 6 and 12 months; (2) patients in the control group who did not maintain a 24-hour urine volume within 0.5 L per day of their baseline value; and (3) patients who missed an assessment or whose final urine sample was collected more than 16 months after randomization. Three post hoc subgroup analyses were conducted for baseline macroalbuminuria, diabetes, and an eGFR below 45 mL/min per 1.73 m2; these analyses were conducted using linear regression with an interaction term.
All analyses except the per-protocol supplementary analysis were conducted according to the intention-to-treat principle. All tests were 2-sided and conducted at the .05 level of significance. Formal adjustment for multiple comparisons was not performed and therefore all analyses of supplementary, secondary, and other outcomes should be considered exploratory. Normally distributed variables were compared using the t test; non-normally distributed variables were compared using the Wilcoxon 2-sample test using the normal approximation, and the 95% CI for the difference between 2 medians was calculated using Hodges-Lehmann estimation. Differences between categorical variables were calculated using the χ2 test, and 95% CIs were calculated with a continuity correction. Statistical analyses were conducted using SAS version 9.3 or later, SPSS version 24 (IBM SPSS), and R (version 3.2.0).
Results
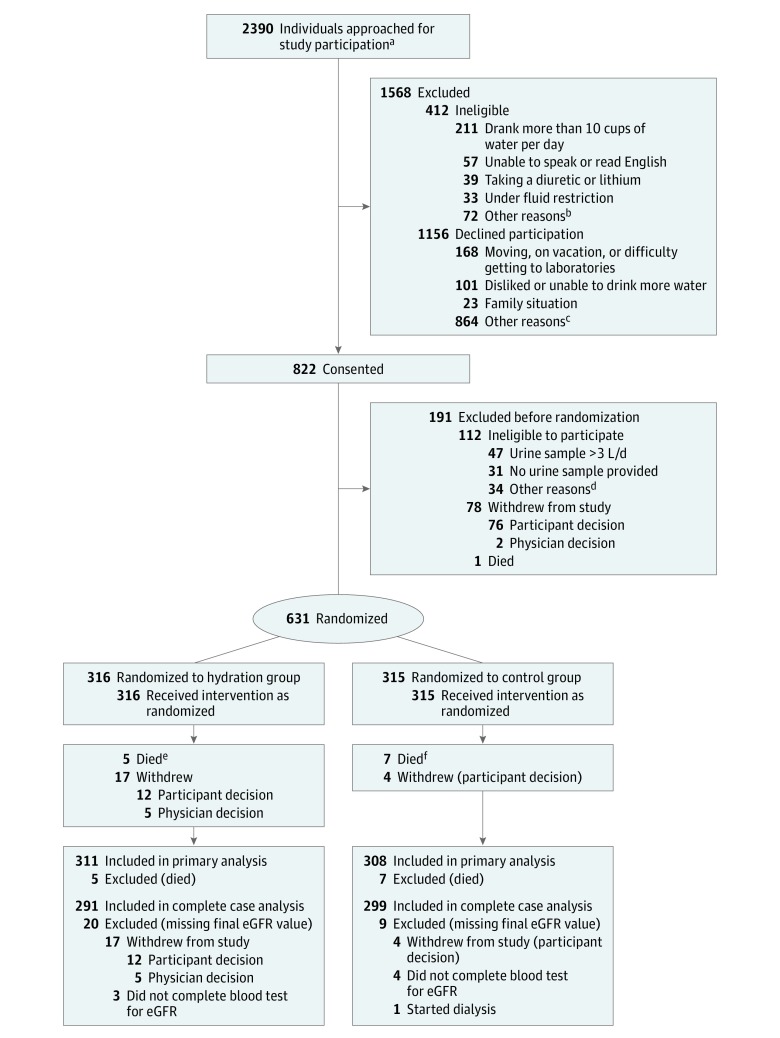
Trial enrollment began on April 8, 2013, and the last patient was randomized on May 6, 2016 . The sample flow is shown in the Figure. Of 2390 patients assessed for eligibility, 1568 did not meet the study’s eligibility criteria or did not wish to participate. Of 822 patients who consented, 191 were excluded before randomization: 76 withdrew for personal reasons, 2 due to physician decision, 1 died, 47 had a 24-hour urine volume above 3 L, 31 failed to provide a 24-hour urine sample, and 34 were ineligible for other reasons (eg, medication contraindications or chemotherapy). The remaining 631 patients were randomized to the hydration group (n = 316) or the control group (n = 315). During the 1-year trial period, 12 of 631 patients died (1.9%) (5 [1.6%] in the hydration group and 7 [2.2%] in the control group); of the 619 survivors, 95% provided 1-year follow-up measurements (last day of follow-up, May 25, 2017).
Figure. Flow of Patients Through the Chronic Kidney Disease Water Intake Trial.
aPrescreened by a research assistant for eligibility.
bOther reasons: no urine protein, enrolled in another study, pregnant or breastfeeding, kidney stones in the past 5 years, less than 2 years of life expectancy, and gastrointestinal issues (eg, inflammatory bowel disease or Crohn disease).
cOther reasons: too busy, did not want to undergo blood tests, did not want to undergo 24-hour urine collections, cancer diagnosis, physician advised not to participate.
dOther reasons: medication contraindications or chemotherapy.
eMedian time to death was 5.1 months (interquartile range, 3.1-7.3).
fMedian time to death was 7.2 months (interquartile range, 2.0-10.2).
eGFR indicates estimated glomerular filtration rate.
Baseline characteristics of 631 randomized patients are shown in Table 1. There were no clinically important differences between randomized groups. Patients had a mean (SD) age of 65.0 years (11.8); 63.4% were men, 90.6% were white, 45.6% were obese (body mass index ≥30), 88.1% had hypertension, and 48.0% had diabetes. The mean (SD) 24-hour urine volume at baseline was 1.9 (0.6) L per day, eGFR was 43.4 (9.8) mL/min per 1.73 m2, and creatinine clearance was 53.5 (17.4) mL/min per 1.73 m2. Median urine albumin was 122.8 mg/d (IQR, 34.9-512.0). At baseline, 150 patients in the control group (47.6% of 315) had a urine volume greater than 1.5 L per day (mean, 2.3 L/d) and a urine osmolality of less than 500 mOsm/kg (mean, 365 mOsm/kg); these patients were asked to decrease their water intake by 1 cup per day.
Table 1. Baseline Characteristics of Patients in the Chronic Kidney Disease Water Intake Trial (N = 631).
| Demographic and Clinical Characteristics | Treatment Group | |
|---|---|---|
| Hydration (n = 316) |
Control (n = 315) |
|
| Age, mean (SD), y | 64.8 (12.0) | 65.2 (11.6) |
| Men, No. (%) | 200 (63.3) | 200 (63.5) |
| Race/ethnicity, No. (%)a | ||
| White | 279 (88.3) | 293 (93.0) |
| South Asian | 14 (4.4) | 3 (1.0) |
| Black | 7 (2.2) | 8 (2.5) |
| Other | 16 (5.1) | 11 (3.5) |
| Weight, mean (SD), kg | 87.2 (18.9) | 87.2 (19.9) |
| BMI,b mean (SD) | 30.0 (6.0) | 29.8 (6.0) |
| Obese (BMI ≥30b), No. (%) | 149 (47.2) | 139 (44.1) |
| Current smoker, No. (%) | 30 (9.5) | 38 (12.1) |
| Daily fluid intake, mean (SD), L/d | 2.1 (0.8) | 2.0 (0.7) |
| Primary etiology of chronic kidney disease, No. (%) | ||
| Hypertension | 127 (40.2) | 119 (37.8) |
| Diabetes | 120 (38.0) | 105 (33.3) |
| Glomerulonephritis | 35 (11.1) | 41 (13.0) |
| Autoimmune | 15 (4.7) | 26 (8.3) |
| Polycystic kidney disease | 9 (2.9) | 9 (2.8) |
| Other/unknown | 10 (3.2) | 15 (4.8) |
| Chronic kidney disease stage, No. (%) | ||
| eGFRc ≥45 mL/min per 1.73 m2 | 141 (44.6) | 148 (47.0) |
| Urine albumin <30 mg/d | 28 (8.9) | 39 (12.4) |
| Urine albumin 30-300 mg/d | 58 (18.4) | 65 (20.6) |
| Urine albumin >300 mg/d | 49 (15.5) | 33 (10.5) |
| eGFRc<45 mL/min per 1.73 m2 | 175 (55.4) | 167 (53.0) |
| Urine albumin <30 mg/d | 31 (9.8) | 33 (10.5) |
| Urine albumin 30-300 mg/d | 77 (24.4) | 63 (20.0) |
| Urine albumin >300 mg/d | 53 (16.8) | 63 (20.0) |
| Comorbidities within last 10 y, No. (%) | ||
| Hypertension | 278 (88.3) | 278 (88.3) |
| Hyperlipidemia | 211 (67.2) | 206 (65.8) |
| Diabetes | 166 (52.7) | 137 (43.5) |
| Malignancy | 60 (19.1) | 72 (23.0) |
| Coronary artery disease | 64 (20.4) | 67 (21.3) |
| Cerebrovascular/transient ischemic attack | 36 (11.5) | 45 (14.3) |
| Peripheral vascular disease | 28 (8.9) | 28 (8.9) |
| Chronic obstructive pulmonary disease | 45 (14.2) | 43 (13.7) |
| Transplant | 13 (4.1) | 20 (6.3) |
| Congestive heart failure | 19 (6.0) | 11 (3.5) |
| Family history of hypertension or kidney failure, No. (%) | 181 (57.3) | 177 (56.2) |
| Systolic blood pressure, mean (SD), mm Hg | 140.8 (19.1) | 136.8 (17.2) |
| Diastolic blood pressure, mean (SD), mm Hg | 79.2 (10.3) | 77.7 (10.9) |
| Measures from blood samples, mean (SD) | ||
| eGFR (creatinine)c, mL/min per 1.73 m2 | 43.2 (10.2) | 43.7 (9.3) |
| eGFR (cystatin C)d, mL/min per 1.73 m2 | 46.7 (19.8) | 47.5 (19.3) |
| Copeptin, pmol/L | 16.6 (11.8) | 17.8 (13.7) |
| Cystatin C, mg/L | 1.6 (0.5) | 1.6 (0.4) |
| Sodium, mEq/L | 139.1 (2.7) | 139.2 (2.6) |
| Osmolality, mOsm/kg | 300.3 (7.4) | 301.2 (8.3) |
| Urea, mg/dL | 28.9 (9.5) | 28.6 (10.4) |
| Measures from 24-h urine samples | ||
| Urine volume, mean (SD), L/d | 1.9 (0.6) | 1.9 (0.6) |
| Creatinine, mean (SD), mmol/d | 11.9 (4.3) | 12.0 (3.7) |
| Osmolality, mean (SD), mOsm/kg | 447.4 (140.0) | 456.6 (132.9) |
| Urea, mean (SD), mmol/d | 339.6 (129.9) | 350.2 (125.7) |
| Sodium, mean (SD), mmol/d | 145.9 (62.4) | 149.2 (62.5) |
| Potassium, mean (SD), mmol/d | 63.3 (26.6) | 63.9 (23.4) |
| Albumin, median (IQR), mg/d | 108 (31-522) | 150 (40-507) |
| Creatinine clearance, mean (SD), mL/min per 1.73 m2 | 53.0 (18.7) | 54.0 (16.4) |
| Medications, No. (%) | ||
| ACE inhibitor or angiotensin receptor blocker | 217 (68.7) | 211 (67.0) |
| Statin | 203 (64.2) | 211 (67.0) |
| Calcium channel blockers | 134 (42.4) | 125 (39.7) |
| β-Blockers | 124 (39.2) | 127 (40.3) |
| Diuretic | 133 (42.1) | 113 (35.9) |
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
SI conversion factors: To convert serum urea to mmol/L, multiply by 0.357.
Self-reported race/ethnicity was collected and recorded by a research assistant using prespecified categories; black/white racial origin is needed for the calculation of eGFR.
BMI was calculated as weight in kilograms divided by height in meters squared.
Calculated using the CKD-EPI creatinine equation.
Calculated using the CKD-EPI cystatin C equation.
Intervention Adherence
The mean 1-year change in 24-hour urine volume was 0.6 L per day greater in the hydration group than the control group (95% CI, 0.5 to 0.7; P < .001), and the mean 9-month change in self-reported fluid intake was 0.7 L per day greater in the hydration group than the control group (95% CI, 0.6 to 0.8; P < .001) (Table 2).
Table 2. Change in 24-Hour Urine Volume and Self-reported Fluid Intakea.
| Mean (SD) | Mean Difference (95% CI)b |
P Valueb | ||
|---|---|---|---|---|
| Hydration Group (n = 291) | Control Group (n = 299) | |||
| Urine volume, L/d | ||||
| Prerandomization | 1.9 (0.6) | 1.9 (0.6) | ||
| 6 Month | 2.5 (0.9) | 1.9 (0.6) | ||
| 12 Month | 2.5 (0.8) | 1.9 (0.7) | ||
| 12-Month change (95% CI) | 0.6 (0.5 to 0.7)c | −0.04 (0.0 to 0.1)c | 0.6 (0.5 to 0.7) | <.001 |
| Self-reported fluid intake, L/d | ||||
| Prerandomization | 2.1 (0.8) | 2.0 (0.7) | ||
| 3 Month | 2.8 (0.9) | 2.0 (0.6) | ||
| 6 Month | 2.8 (0.8) | 2.1 (0.7) | ||
| 9 Month | 2.8 (0.8) | 2.0 (0.6) | ||
| 9-Month change (95% CI) | 0.7 (0.6 to 0.8)c | 0.0 (−0.1 to 0.1)c | 0.7 (0.6 to 0.8) | <.001 |
All analyses followed the intention-to-treat principle.
Between-group differences in change were analyzed using the independent-samples t test.
Change was calculated as the final value minus the prerandomization value; restricted to patients who provided follow-up data within 8 to 16 months of randomization (excluding 12 patients who died during the 12-month trial period [5 in the hydration group and 7 in the control group]).
Primary Outcome: Change in eGFR
A scatterplot of the change in eGFR by baseline levels in the hydration and control groups is shown in eFigure 1 in Supplement 3. The mean 1-year decline in eGFR was −2.2 mL/min per 1.73 m2 in the hydration group and −1.9 mL/min per 1.73 m2 in the control group; the adjusted between-group difference in change was −0.3 mL/min per 1.73 m2 (95% CI, −1.8 to 1.2; P = .74) (Table 3).
Table 3. Primary Outcome: 1-Year Change in Estimated Glomerular Filtration Ratea.
| eGFR, mL/min per 1.73 m2 | Mean (95% CI) | Adjusted Between-Group Difference in Changeb (95% CI) | P Value | |
|---|---|---|---|---|
| Hydration Group (n = 311) |
Control Group (n = 308) |
|||
| Prerandomization | 43.3 (42.1 to 44.4) | 43.6 (42.6 to 44.7) | ||
| 12 Months | 41.0 (39.5 to 42.6) | 41.7 (40.3 to 43.1) | ||
| Change | −2.2 (−3.3 to −1.1)c | −1.9 (−2.9 to −0.9)c | −0.3 (−1.8 to 1.2) | .74 |
Abbreviation: eGFR, estimated glomerular filtration rate.
Analyses exclude 12 patients who died during the 12-month trial period (5 in the hydration group; 7 in the control group); all analyses followed the intention-to-treat principle. Fully conditionally specified models were used to impute missing 12-month eGFR values for 29 patients.
The adjusted between-group difference in eGFR change was analyzed using linear regression, adjusted for age (in years), sex, obesity (body mass index ≥30 [calculated as weight in kilograms divided by height in meters squared]), current smoker, presence of diabetes, urinary albumin (mg/d) (log-transformed), and use of the following medications: an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, diuretic, β-blocker, calcium channel blocker, and statin.
The mean change in eGFR (12-month value minus the prerandomization value) was calculated within each of 20 imputed data sets, and the mean was calculated using the imputed data set means.
Results were similar in the unadjusted analysis and when eGFR was calculated using cystatin C (eTable 6 in Supplement 3), when eGFR change was analyzed longitudinally (eAppendix 2 in Supplement 3), and in a mixed-effects model accounting for the effect of center (eTable 7 in Supplement 3). The mean percentage decline in eGFR from baseline was 4.6% in the hydration group and 4.4% in the control group (difference, −0.2 [95% CI, −3.8 to 3.5]; P = .93), and the proportion of patients with an eGFR decline of at least 20% over 1 year was 21.6% in the hydration group and 20.7% in the control group (difference, 0.9% [95% CI, −5.7% to 7.5%]; P = .79) (eTable 8 in Supplement 3).
Per-Protocol Analysis
Of 316 patients in the hydration group, 89 (28.2%) maintained a urine volume that was at least 0.5 L per day greater than their baseline value at each follow-up assessment. Of 315 patients in the control group, 184 (58.4%) had a urine volume that remained at least 0.5 L per day less than their baseline value at each follow-up assessment. In these per-protocol groups, the 1-year change in 24-hour urine volume was 1.5 L per day more in the hydration group than in the control group (95% CI, 1.3 to 1.6; P < .001), and the mean 1-year change in eGFR was −1.5 mL/min per 1.73 m2 in the hydration group vs −1.6 mL/min per 1.73 m2 in the control group; the between-group difference was 0.2 mL/min per 1.73 m2 (95% CI, −1.9 to 2.3; P = .86).
Subgroup Analyses
No statistically significant interactions were observed in 3 post hoc subgroup analyses of patients with and without macroalbuminuria (eTable 9 in Supplement 3) or diabetes (eTable 10 in Supplement 3), and in those with an eGFR of at least 45 mL/min per 1.73 m2 or less than 45 mL/min per 1.73 m2 at baseline (eTable 11 in Supplement 3).
Secondary Outcomes
As shown in Table 4, statistically significant between-group differences were seen for plasma copeptin concentration and creatinine clearance but not for 24-hour urine albumin or patient-reported quality of health. The 1-year change in plasma copeptin concentration was 2.2 pmol/L lower in the hydration group than the control group (95% CI, −3.9 to −0.5; P = .01), and the 1-year change in creatinine clearance was 3.6 mL/min per 1.73 m2 higher in the hydration group than the control group (95% CI, 0.8 to 6.4; P = .01). The between-group difference in the 1-year change in urine albumin was 6.8 mg/d (95% CI, −3.9 to 50.8; P = .11), and the between-group difference in the 1-year change in quality of life was 0.2 units (95% CI, −0.3 to 0.3; P = .22).
Table 4. Secondary Outcomes: 1-Year Change in Plasma Copeptin, Creatinine Clearance, 24-Hour Urine Albumin, and Patient-Reported Overall Quality of Healtha.
| Hydration Group (n = 291) |
Control Group (n = 299) |
Between-Group Difference in Changeb (95% CI) |
P Valueb | |
|---|---|---|---|---|
| Plasma copeptin, mean (SD), pmol/L | ||||
| Prerandomization | 17.4 (12.4) | 17.5 (13.0) | ||
| 12 Month | 16.1 (13.5) | 18.3 (13.6) | ||
| Change (95% CI) | −1.4 (−2.7 to −0.0)c | 0.8 (−0.3 to 1.9)c | −2.2 (−3.9 to −0.5) | .01 |
| Creatinine clearance, mean (SD), mL/min per 1.73 m2 | ||||
| Prerandomization | 52.5 (17.1) | 54.6 (15.6) | ||
| 12 Month | 53.1 (19.7) | 51.6 (18.9) | ||
| Change (95% CI) | 0.6 (−1.6 to 2.7)c | −3.0 (−4.9 to −1.2)c | 3.6 (0.8 to 6.4) | .01 |
| Urine albumin, mean (SD), mg/d | ||||
| Prerandomization | 139 (49 to 425) | 108 (32 to 606) | ||
| 12 Month | 142 (38 to 509) | 103 (32 to 497) | ||
| Change (95% CI) | 7 (−4.1 to 16.2)c | 0.2 (−7.0 to 4.6)c | 6.8 (−3.9 to 50.8) | .11 |
| Quality of healthd | ||||
| Prerandomization | 7.3 (1.6) | 7.3 (1.6) | ||
| 12 Month | 7.2 (1.9) | 7.1 (1.8) | ||
| Change (95% CI) | −0.0 (−0.3 to 0.2)c | −0.2 (−0.4 to −0.0)c | 0.2 (−0.3 to 0.3) | .22 |
Abbreviation: IQR, interquartile range.
All analyses followed the intention-to-treat principle. No adjustment was made for multiple comparisons; therefore all results should be interpreted as exploratory.
Between-group differences in change were analyzed using the independent-samples t test (for normally distributed data) or the Wilcoxon 2-sample test using the normal approximation (for non-normally distributed data); CIs for the difference between 2 medians were calculated using Hodges-Lehmann estimation.
Change was calculated as the 12-month value minus the prerandomization value; restricted to patients who provided follow-up data within 8 to 16 months of randomization (excluding 12 patients who died during the 12-mo trial period [5 in the hydration group and 7 in the control group]).
Measured on a 10-point Likert scale (0 [worst possible] to 10 [best possible]).
Other Outcomes
The mean 1-year change in 24-hour urine osmolality, creatinine, urea, sodium, and potassium are shown in eTable 12 in Supplement 3; between-group differences were −75.6 mOsm/kg (95% CI, −98.2 to −53.0; P < .001) for urine osmolality, 0.8 mmol per day (95% CI, 0.3 to 1.4; P = .003) for creatinine, 25.4 mmol per day (95% CI, 5.1 to 45.6; P = .01) for urea, 21.9 mmol per day (95% CI, 10.3 to 33.6; P < .001) for sodium, and 2.8 mmol per day (95% CI, −1.6 to 7.1; P = .22) for potassium. No statistically significant differences were seen between groups for the 1-year change in serum osmolality, creatinine, or urea (eTable 13 in Supplement 3); blood pressure, weight, waist circumference, or body mass index (eTable 14 in Supplement 3); or the 6-month change in dietary intake of protein and sodium (eTable 15 in Supplement 3).
Adverse Events and Serum Sodium Monitoring
The data and safety monitoring board performed 5 reviews of trial safety and integrity during the recruitment period and recommended that the trial continue. During the trial, 52 adverse events occurred among 42 (13%) patients in the hydration group (including 5 deaths [3 additional deaths occurred between 13 and 16 months]) and 66 events occurred among 51 (16%) patients in the control group (including 7 deaths [3 additional deaths occurred between 13 and 16 months]). All adverse events were investigated, and no events were attributed to the intervention. Patients’ serum sodium levels were monitored also at 3-month intervals (eTable 16 in Supplement 3). Serum sodium concentration declined to less than 130 mEq/L in 3 patients in the hydration group and in 1 participant in the control group; each case was investigated and reported to the participant’s physician; all values normalized by the next study visit. The 1-year change in serum sodium was 0.5 mEq/L lower in the hydration group than the control group (95% CI, −1.0 to −0.04; P = .04).
Discussion
In this randomized clinical trial of approximately 600 patients with stage 3 chronic kidney disease, drinking more water (compared with usual fluid intake alone) did not slow the decline in eGFR over 1 year. This trial had 80% statistical power to detect a between-group difference in eGFR change of 2 mL/min per 1.73 m2. Findings from the primary intention-to-treat analyses were consistent with the per-protocol analyses for which there was a greater separation in 24-hour urine volume between groups.
The absence of an effect of drinking more water on eGFR may be interpreted in several ways. First, increased water intake may not protect against declining kidney function, and the results of prior observational studies may have been confounded.9,10,11
Second, there may have been an effect, but the study was underpowered to identify it as statistically significant (this study was underpowered to detect a between-group difference less than 2 mL/min per 1.73 m2); however, the 1-year decline in eGFR was slightly greater in the hydration group, suggesting a possible effect in the opposite direction to that originally hypothesized.
Third, although the hydration group achieved a significantly increased fluid intake and decreased copeptin concentrations relative to the control group, perhaps a greater difference in water intake is needed to produce a measurable effect on eGFR (although the per-protocol analysis was consistent with the intent-to-treat analysis).
Fourth, more than 1 year of follow-up may be needed to detect an effect on eGFR. In a clinical trial of patients with autosomal dominant polycystic kidney disease treated with Tolvaptan (a vasopressin V2-receptor antagonist) vs placebo, between-group differences in kidney function did not emerge until 24 to 36 months after randomization.38 Similarly, a 7-year observational study of 2000 healthy adults showed less eGFR decline with larger urine outputs.9 Although patients in the present trial will undergo an additional 12 months of follow-up to evaluate 2-year eGFR change, hydration coaching stopped at 12 months, which may negatively affect patients’ adherence to their random allocation.16
A marked incongruence was observed between the 1-year change in eGFR and creatinine clearance. The between-group difference in the 1-year change in GFR estimated with serum creatinine was −0.3 mL/min per 1.73 m2 and with serum cystatin C was −0.2 mL/min per 1.73 m2 (P > .05); however, the 1-year change in creatinine clearance was significantly higher in the hydration group relative to the control group (the between-group difference was 3.5 mL/min per 1.73 m2 [P = .01]). The latter finding appeared to be driven by an increased urinary excretion of creatinine in the hydration group relative to the control group (eTable 12 in Supplement 3). Three explanations for this finding were considered. Given that the urinary excretion of urea and sodium were also significantly increased in the hydration group relative to the control group (eTable 12 in Supplement 3), the possibility of systematic urine collection errors was considered; however, urine osmolality and plasma copeptin both decreased significantly more in the hydration group relative to the control group, suggesting that patients were following their randomly assigned intervention. In addition, an increased urinary excretion of creatinine, urea, and sodium may reflect an increase in weight (eTable 14 in Supplement 3) or intake of protein and sodium (eTable 15 in Supplement 3); however, no between-group differences in these variables were observed, although perhaps there were dietary changes that went unrecorded. Also, it is possible that patients in the hydration group had an increased urinary flow rate, which has been shown to cause increased tubular secretion and decreased reabsorption of urinary solutes (specifically creatinine and urea).39,40 The clinical significance of an increased urinary flow rate is unknown and further research is needed to better understand this finding and the underlying physiology.
Limitations
This study has several limitations. First, despite the efforts of dieticians and research assistants who coached patients in the hydration group to drink 1.0 to 1.5 L of water per day (depending on sex and weight), mean urine volume in the hydration group increased by only 0.6 L per day (2.4 cups) relative to the control group. This highlights how difficult it would be to achieve a large sustained increase in water intake in routine practice. However, the increased water intake achieved in this trial was sufficient to lower vasopressin secretion, as assessed by plasma copeptin concentrations (the 1-year change in plasma copeptin was 2.3 pmol/L lower in the hydration group than the control group [P = .009]).
Second, although the magnitude of eGFR decline was 0.3 mL/min per 1.73 m2 greater in the hydration group relative to the control group, the 95% CI around this estimate spanned −1.8 to 1.2, and the study was underpowered to detect the prespecified minimal clinically important difference of 1 mL/min per 1.73 m2; therefore, trial findings should be interpreted as null but potentially underpowered.
Third, the generalizability of trial findings may be limited by enrollment restricted to patients in Southwestern Ontario, Canada (a northern region with a temperate climate) in which 90% of the patients were white.
Fourth, the use of estimated rather than measured GFR represents an important source of measurement error in this study. Exogenously measured GFR may be more sensitive to changes in kidney function; however, measurement protocols are more invasive and costly, which may be considered unacceptable to patients.
Conclusions
Among adults with stage 3 chronic kidney disease, coaching to increase water intake compared with coaching to maintain the same water intake did not significantly slow the decline in kidney function after 1 year. However, the study may have been underpowered to detect a clinically important difference.
Trial Protocol
Statistical Analysis
eFigure 1. Scatterplot of the change in estimated glomerular filtration rate by baseline levels in the hydration and control groups
eTable 1. Justification of changes made to the original trial protocol
eTable 2. Summary of outcomes analyzed in the Chronic Kidney Disease Water Intake Trial (CKD WIT)
eTable 3. Eligibility criteria for the Chronic Kidney Disease Water Intake Trial (CKD WIT)
eTable 4. Hydration intervention by age and sex
eTable 5. Schedule of study visits and measures
eTable 6. Post-hoc analyses of the unadjusted one-year change in eGFR using the CKD-EPI creatinine and cystatin C equations
eTable 7. Post-hoc analyses of the adjusted one-year change in eGFR using a mixed-effects model
eTable 8. Percentage change in eGFR
eTable 9. Post-hoc subgroup analysis of the one-year change in eGFR in participants with and without macroalbuminuria at baseline
eTable 10. Post-hoc subgroup analysis of the one-year change in eGFR in participants with and without diabetes at baseline
eTable 11. Post-hoc subgroup analysis of the one-year change in eGFR in participants with a baseline eGFR above (>) or below 45 mL/min per 1.73m2
eTable 12. Post-hoc analyses of the one-year change in 24-hour urine osmolality, creatinine, urea, sodium, and potassium
eTable 13. Post-hoc analyses of the one-year change in serum osmolality, creatinine, and urea
eTable 14. One-year change in blood pressure, weight, waist circumference, and body mass index
eTable 15. Post-hoc analyses of self-reported dietary intake of sodium and protein
eTable 16. One-year change in serum sodium
eAppendix 1.
eAppendix 2.
eReferences.
References
- 1.Food and Nutrition Board—National Academy of Sciences Recommended Dietary Allowances. Natl Res Counc. 1945;No. 122:3-18.
- 2.Valtin H. “Drink at least eight glasses of water a day.” really? is there scientific evidence for “8 x 8”? Am J Physiol Regul Integr Comp Physiol. 2002;283(5):R993-R1004. [DOI] [PubMed] [Google Scholar]
- 3.Vreeman RC, Carroll AE. Medical myths. BMJ. 2007;335(7633):1288-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lette F, Dwyer JP. The fluid craze. Lancet. 2008;372(9641):782-784. [DOI] [PubMed] [Google Scholar]
- 5.Clark WF, Sontrop JM, Huang S-H, Moist L, Bouby N, Bankir L. Hydration and chronic kidney disease progression: a critical review of the evidence. Am J Nephrol. 2016;43(4):281-292. [DOI] [PubMed] [Google Scholar]
- 6.Wang CJ, Grantham JJ, Wetmore JB. The medicinal use of water in renal disease. Kidney Int. 2013;84(1):45-53. [DOI] [PubMed] [Google Scholar]
- 7.Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol. 2013;9(4):223-239. [DOI] [PubMed] [Google Scholar]
- 8.Bouby N, Bachmann S, Bichet D, Bankir L. Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol. 1990;258(4 Pt 2):F973-F979. [DOI] [PubMed] [Google Scholar]
- 9.Clark WF, Sontrop JM, Macnab JJ, et al. . Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol. 2011;6(11):2634-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sontrop JM, Dixon SN, Garg AX, et al. . Association between water intake, chronic kidney disease, and cardiovascular disease: a cross-sectional analysis of NHANES data. Am J Nephrol. 2013;37(5):434-442. [DOI] [PubMed] [Google Scholar]
- 11.Strippoli GF, Craig JC, Rochtchina E, Flood VM, Wang JJ, Mitchell P. Fluid and nutrient intake and risk of chronic kidney disease. Nephrology (Carlton). 2011;16(3):326-334. [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians . Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161(9):659-667. [DOI] [PubMed] [Google Scholar]
- 13.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol. 1996;155(3):839-843. [PubMed] [Google Scholar]
- 14.Clark WF, Sontrop JM, Huang SH, et al. . The chronic kidney disease Water Intake Trial (WIT): results from the pilot randomised controlled trial. BMJ Open. 2013;3(12):e003666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sontrop JM, Huang S-H, Garg AX, et al. . Effect of increased water intake on plasma copeptin in patients with chronic kidney disease: results from a pilot randomised controlled trial. BMJ Open. 2015;5(11):e008634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark WF, Huang S-HS, Garg AX, et al. . The Chronic Kidney Disease Water Intake Trial (WIT): protocol of a randomized controlled trial[published online August 22, 2017]. Can J Kidney Heal Dis. doi: 10.1177/2054358117725106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295-309. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-S266. [PubMed] [Google Scholar]
- 19.Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol. 2011;6(1):192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barash I, Ponda MP, Goldfarb DS, Skolnik EY. A pilot clinical study to evaluate changes in urine osmolality and urine cAMP in response to acute and chronic water loading in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5(4):693-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spigt MG, Knottnerus JA, Westerterp KR, Olde Rikkert MG, Schayck CP. The effects of 6 months of increased water intake on blood sodium, glomerular filtration rate, blood pressure, and quality of life in elderly (aged 55-75) men. J Am Geriatr Soc. 2006;54(3):438-443. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christ-Crain M, Fenske W. Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol. 2016;12(3):168-176. [DOI] [PubMed] [Google Scholar]
- 24.Hays RD, Kallich J, Mapes D, et al. . Kidney Disease Quality of Life Short Form (KDQOL-SF TM), version 1.3. 1997. http://www.rand.org/pubs/papers/P7994.html. Accessed January 12, 2017.
- 25.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3(5):329-338. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20(12):2617-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turin TC, Coresh J, Tonelli M, et al. . One-year change in kidney function is associated with an increased mortality risk. Am J Nephrol. 2012;36(1):41-49. [DOI] [PubMed] [Google Scholar]
- 28.Roussel R, Fezeu L, Marre M, et al. . Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab. 2014;99(12):4656-4663. [DOI] [PubMed] [Google Scholar]
- 29.Enhörning S, Christensson A, Melander O. Plasma copeptin as a predictor of kidney disease[published online February 16, 2018]. Nephrol Dial Transplant. doi: 10.1093/ndt/gfy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cravedi P, Ruggenenti P, Remuzzi G. Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol. 2012;8(5):301-306. [DOI] [PubMed] [Google Scholar]
- 31.Rosansky SJ. Renal function trajectory is more important than chronic kidney disease stage for managing patients with chronic kidney disease. Am J Nephrol. 2012;36(1):1-10. [DOI] [PubMed] [Google Scholar]
- 32.Eriksen BO, Tomtum J, Ingebretsen OC. Predictors of declining glomerular filtration rate in a population-based chronic kidney disease cohort. Nephron Clin Pract. 2010;115(1):c41-c50. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzo V, Saracho R, Zamora J, Rufino M, Torres A. Similar renal decline in diabetic and non-diabetic patients with comparable levels of albuminuria. Nephrol Dial Transplant. 2010;25(3):835-841. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Hopewell S, Schulz KF, et al. . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy J, Shou H, Xie D, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . Statistical methods for cohort studies of CKD: prediction modeling. Clin J Am Soc Nephrol. 2017;12(6):1010-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76(12):1049-1064. doi: 10.1080/10629360600810434. [DOI] [Google Scholar]
- 37.Coresh J, Turin TC, Matsushita K, et al. . Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres VE, Chapman AB, Devuyst O, et al. ; TEMPO 3:4 Trial Investigators . Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjöström PA, Odlind BG, Wolgast M. Extensive tubular secretion and reabsorption of creatinine in humans. Scand J Urol Nephrol. 1988;22(2):129-131. [DOI] [PubMed] [Google Scholar]
- 40.Joppich R, Deetjen P. The relation between the reabsorption of urea and of water in the distal tubule of the rat kidney. Pflugers Arch. 1971;329(2):172-185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis
eFigure 1. Scatterplot of the change in estimated glomerular filtration rate by baseline levels in the hydration and control groups
eTable 1. Justification of changes made to the original trial protocol
eTable 2. Summary of outcomes analyzed in the Chronic Kidney Disease Water Intake Trial (CKD WIT)
eTable 3. Eligibility criteria for the Chronic Kidney Disease Water Intake Trial (CKD WIT)
eTable 4. Hydration intervention by age and sex
eTable 5. Schedule of study visits and measures
eTable 6. Post-hoc analyses of the unadjusted one-year change in eGFR using the CKD-EPI creatinine and cystatin C equations
eTable 7. Post-hoc analyses of the adjusted one-year change in eGFR using a mixed-effects model
eTable 8. Percentage change in eGFR
eTable 9. Post-hoc subgroup analysis of the one-year change in eGFR in participants with and without macroalbuminuria at baseline
eTable 10. Post-hoc subgroup analysis of the one-year change in eGFR in participants with and without diabetes at baseline
eTable 11. Post-hoc subgroup analysis of the one-year change in eGFR in participants with a baseline eGFR above (>) or below 45 mL/min per 1.73m2
eTable 12. Post-hoc analyses of the one-year change in 24-hour urine osmolality, creatinine, urea, sodium, and potassium
eTable 13. Post-hoc analyses of the one-year change in serum osmolality, creatinine, and urea
eTable 14. One-year change in blood pressure, weight, waist circumference, and body mass index
eTable 15. Post-hoc analyses of self-reported dietary intake of sodium and protein
eTable 16. One-year change in serum sodium
eAppendix 1.
eAppendix 2.
eReferences.